Research - Der Pharmacia Lettre ( 2022) Volume 14, Issue 5
Received: 13-Jun-2022, Manuscript No. DPL-22-66435; Editor assigned: 17-Jun-2022, Pre QC No. DPL-22-66435(PQ); Reviewed: 01-Jul-2022, QC No. DPL-22-66435; Revised: 07-Jul-2022, Manuscript No. DPL-22-66435(R); Published: 14-Jul-2022 , DOI: 10.37532/dpl.2022.14.16
Objectives: The study's goal was to determine the phytochemical elements and antioxidant potential in the leaves of Syzygium cumini (Myrtaceae) (using a variety of polarity solvents, including aqueous, ethanol, and methanol), which have traditional and complementary medicines to treat a variety of metabolic problems.
Methodology: The leaves were washed, dried in the open air and ground into a coarse powder. Before the phytochemical screening, the powders were separated into five groups and soaked individually for one week in distilled water, ethanol, methanol, ethyl acetate, and hexane.
Results: The antioxidant activity ranged from 12 to 20 mg/ml with the 50 percent ethanol extract having the maximum antioxidant activity. The sample’s phenolic concentration was found to be between 0.54 and 1.12 mg/ml. The aqueous extract had the highest value (50 percent). The reducing sugar content for the sample was determined to be between 1.29 to 2.02 mg/ml. In the ethanolic extract, the greatest value was found. The alkaloids content ranged from 2.05 to 6.15 mg/gm, with the ethanolic extract containing the most. The protein level of the leaf sample ranged from 1.5 to 7.5 mg/ml. In the case of methanolic acid, the highest value was found (50 percent).
Conclusion: Plants include phytochemicals with antibacterial action, such as alkaloids, phenol, and essential oils. This is why herbs have been used to promote oral hygiene and prevent the emergence of common ailments since antiquity. Herbs and herbal products will play an increasingly important role in health and hygiene in the future.
Syzygium cumini (L.) Leaves extracts, Phytochemical analysis, Antioxidant assay.
Syzygium cumini often known as duhat, black plum, or Java plum, is a popular fruit that can be found all across the Philippines. It is both planted and natural in various areas. India, Myanmar, Sri Lanka, Thailand, Australia, Colombia, Cuba, Mexico, Nepal, Kenya, the United States of America, Zambia, and Zimbabwe are all home to this species. Duhat is an evergreen tree with greyish white stems and coarse, discolored bottom bark that grows up to 25 meters (80 feet) tall. Simple, opposite, elliptic to oblong, smooth, shiny, and slightly leathery leaves. The leaves are also between 5 and 10 cm (1.2 inches) long. The yellowish midrib of the leaves is noticeable. The leaf blades have several parallel lateral veins. Flowers range in color from white to pinkish is about 1 centimeter (0.5 inch) wide, and has four petals and numerous stamens. The calyx is shaped like a cup. The fruits are ovoid 1 seeded berry that measure 2 centimeters (0.8 inch) in length, are dark purple-red in color, lustrous, and have white to lavender flesh [1-8]. Tannins, alkaloids, flavonoids, sterols, glycosides, and carbohydrates were found in ethanolic extracts of S cumini leaves, while flavonoids were found in methanolic extracts [7]. Saponins and flavonoids were found in greater abundance in the ethyl acetate and methanol extracts of seeds than alkaloids, glycosides, triterpenoids, steroids, and tannins [3]. Tannins were also found in the bark (13.4 percent) [5].
Because of the abundance of Jamun trees, the Indian subcontinent is known as Jambudweep in Indian mythology. The whole plant is traditionally used for its multiple advantages. Despite its ethno pharmacological usage, the presence of several phytoconstituents such as acids, alkyl alcohol, essential oils, flavanol glycosides, hydrocarbon, sugar, polyphenol, and phytosterols has led to the discovery of many therapeutically useful uses for leaves [9,10]. The leaves are used to treat leucorrhoea, stomachaches, fevers, dermopathy, constipation, and to reduce radiation-induced DNA damage [10]. They also limit blood discharges in the feces and minimize radiation-induced DNA damage. Identification, adulteration detection, and quality assessment, which are directly linked to efficacy, require pharmacognostic and physicochemical examination of medicinal plants. Evaluating medicinal plants' plants’ quality is critical to justify its acceptance in the current era. The lack of stringent quality control profiles for herbal products is one of the primary issues confronting the herbal business [11]. There isn’t a single publication on the pharmacognostic evaluation of S.cumini leaf, according to the literature search. As a result, comprehensive research of pharmacognostic standardization was conducted following API and WHO standards and guidelines for medicinal plants.Phytochemical study of medicinal plants is crucial and profitable for both research institutes and pharmaceutical corporations in developing novel medications to cure a variety of disorders. This study aimed to look into a preliminary phytochemical screening of Syzygium cumini seeds from the Myrtaceae family. The presence of medicinally relevant phytochemical elements in the ethyl acetate and methanol extracts of Syzygium cumini seeds was discovered, supporting their use in traditional medicines for the treatment of various disorders [12]. The leaves of Syzygium cumini were subjected to a phytochemical analysis using crude methanol and aqueous extracts (L.) Using the disc diffusion method, the extract’s antibacterial activity was tested against standard strains and clinical isolates of various bacteria. Flavonoids, alkaloids, glycosides, steroids, phenols, saponins, terpenoids, cardiac glycosides, and tannins were identified as chemical classes contained in the extracts in preliminary phytochemical analyses. Salmonella enteritidis, Salmonella typhi, Salmonella typhi A, Salmonella paratyphi A, Salmonella paratyphi B, Pseudomonas aeruginosa, and Escherichia coli clinical isolates all showed inhibitory activity against the extracts. Gram-positive bacteria include Bacillus subtilis and Staphylococcus aureus. The methanol extracts were found to be more powerful than the aqueous extracts [12].Medicinal plants are a local heritage with global significance, and the world is richly supplied with medicinal plants [13]. Medicinal plants are the most abundant bio-resource of traditional medicine pharmaceuticals, contemporary medicine nutraceuticals, food supplements, folk medicine, pharmaceutical intermediates, and chemical entities for synthesized drugs [14]. Phytochemicals are compounds found naturally in medicinal plants, leaves, vegetables, and roots that act as a defensive mechanism and protect against a variety of diseases [15]. Syzygium cumini (L.) belongs to the Myrtaceae family. Large trees cultivated for their edible fruits (Black Plum) throughout India are said to contain vitamin C, gallic acid, tannins, anthocyanins (cyanidin, petunidin, malvidin glucoside, and other components) [16]. (Wealth of India Raw materials 1976). Syzygium cumini is a medicinal plant whose components have been shown to have hypoglycaemic, antimicrobial, anti-HIV activity and anti-diarrhea properties in pharmacological studies. The leaves of this plant were traditionally used as an astringent to alleviate fever and to stop diarrhea [17]. Many articles have claimed that the leaves extract of this plant has pharmacological activity such as antibacterial, anti-diabetic, immunomodulatory, and anticancer properties [18-21]. Triterpenoids and flavonoids were discovered in the leaves of S. samarangense in a previous phytochemical investigation [19,22, 23].
Plant tissue culture as a source of phytochemicals on a continuous basis
S. cumini fruits have a high anthocyanin content, which contributes to their antioxidant and free radical scavenging characteristics. For the food processing industry, these pigments could be a potential source of natural food colorants. However, because the fruit is seasonal, there is a limit to the continual supply hence an in vitro system for pigment production throughout the year must be constructed. Tissue culture mass propagation of forest trees is extremely challenging. Various scientific groups have made numerous attempts to cultivate this tree using tissue culture [24,25]. The bulk of woody plants are difficult to propagate through vegetative methods. In addition, S. cumini has poor seed viability and germination in its natural environment [26]. The seed germinates when new, but it is no longer viable after two weeks at room temperature [27]. Seeds germinate effectively while they are new, but their viability deteriorates after two weeks of open storage at room temperature. Explants turn brown quickly after inoculation because they release large amounts of polyphenols into the culture media. For large-scale in vitro production of the pigments from this tree, stable colored callus and suspension cultures must be produced. For usage as a natural colorant, the pigment can be tested for its stability to various physical factors. Using nodal or meristem explants from mature trees, the tree was successfully cloned using the micro propagation technique [28-30]. Controlling the browning of the culture media with phenolic is easier with in vitro produced seedlings as explants for micro propagation. In ancient Indian literature, the medicinal characteristics of various herbal plants were chronicled, and the concoctions were found to be beneficial in treating ailments. As a result, the need for medicinal plants has skyrocketed to fulfill the rising demand for contemporary medicine manufacturing and export. Uprooted medicinal herbs are commonly used to meet this demand [31]. The discovery rate of new antimicrobial medications has decreased in the last ten years, but the prevalence of resistance has skyrocketed [32]. The problem of germ resistance is becoming more prevalent, and the future usage of antimicrobial treatments remains questionable. As a result, steps must be made to mitigate the problem, such as limiting antibiotic use and doing research to better understand the genetic mechanisms of resistance. His motivated us to investigate plants as a possible source of chemotherapeutic, antibacterial, and ethno medicinal agents [33].
Plant material
The leaves of Jamun/Syzygium cumini were taken and authenticated By Dr. Tripti Bhatnagar at Codon Biotech Pvt. Ltd., Noida-India, and shade dried (totally inside the oven for 48 hours), powdered, and extracted in a soxhlet apparatus with methanol and aqueous, respectively due to their polarity in nature. The hexane and aqueous extracts were filtered through what man number 1 filter paper after extraction and kept for further use
Preliminary phytochemical screening
Alcoholic (Methanol) (Me-OH) and aqueous extract of S. cumini were prepared by cold maceration method. Coarsely powdered air-dried material 5 g was accurately weighed and placed in a glass stoppered conical flask. Put 15 ml distilled water and mix it properly. Covered the beaker with foil and incubated at 50°C for 48 hours.
After incubation, transferred the mixture in centrifuge tubes and centrifuged at 10,000 rpm for 15 minutes. Collected 10 ml supernatant and stored it under refrigeration. 50% extract of ethanol, methanol, and aqueous was prepared. Both the extracts were subjected to phytochemical screening for qualitative analysis for the presence and absence of secondary metabolites.
Phytochemical analysis
The preliminary qualitative phytochemical analyses of alkaloids, phenols, reducing sugar, protein, antioxidant assay, and gallic acid in the Syzygium cumini leaf extracts were carried out using the standard methods as described before. For alkaloid (Bromocresol green method), Reducing sugar (Dinitro salicyclic Acid method), Protein Estimation (Biurets test), Antioxidant assay (Potassium ferrocyanide reducing method), Gallic Acid Estimation (By Folin’s reagent and Sodium bicarbonate) etc. were conducted and the absorbance of the reaction medium was monitored by spectrophotometer
Estimation of alkaloid
The alkaloid estimation was done by the Bromo Cresol Green (BCG) method using atropine as standard. Aliquots of the extract were taken in glass tubes and made up to 7 ml using phosphate buffer. Then 5 ml of BCG and 4 ml of chloroform was added to each tube and left for 20 min. The lower layer of chloroform was removed, and the absorbance was measured at 470 nm using a spectrophotometer.
Determination of total phenol
Total phenol content of the extracts was determined according to the method described by Malik and Singh using gallic acid as standard. Aliquots of the extracts were taken in a 10 ml glass tube and made up to a volume of 2 ml with distilled H2O. Then the 0.1 ml folinciocalteau reagent (1:1 with water) and 0.02 ml of phenol, and 2 ml NaHCO3 (20%) were added sequentially in each tube. The tubes with the solution were warmed for 1 minute and then cooled.
Because the phenols undergo a complex redox reaction with phosphomolybdic acid in folin ciocalteau reagent in an alkaline media, resulting in a blue colored complex, each tube developed a blue color. The absorbance was measured at 765 nm in a spectrophotometer. Total phenol content of the extracts was determined according to the method described by Malik and Singh using Gallic acid as standard.
Estimation of reducing sugar using DNS test
The estimation of reducing sugar was done by Dinitro Salicylic Acid (DNS) method using maltose as standard. Aliquots of the extracts were taken in glass tubes and made up to a volume of 1 ml with distilled water. 2 ml of DNS was added and vortexed. The tubes were heated in a boiling water bath for 5 min at 60°C, and after cooling 1 ml of 40% potassium sodium tartarated and 6 ml distilled water were added. The absorbance was measured at 540 nm by using a spectrophotometer.
Protein quantification
Protein was estimated by Biurret test. The biuret test is a chemical test used for detecting the presence of peptide bonds. In the presence of peptides, a copper (II) ion forms a violet-colored complex in an alkaline solution. Several variants on the test have been developed. Because peptide bonds form with roughly the same frequency per gram of material (for most proteins), the Biuret reaction can be used to determine protein concentration. According to the Beer-Lambert equation, the intensity of the color, and thus the absorption at 540 nm, is exactly proportional to the protein concentration. A peptide bond links the amino group and the carboxyl acid group on adjacent amino acids in a protein. When the Biuret reagent is applied to a peptide bond-containing solution, the result is a violet-colored solution. The violet color is a positive test for the presence of protein the more intense the color, the greater the number of peptide bonds present. Potassium hydroxide (KOH) and hydrated copper (II) sulfate, as well as potassium sodium tartrate, make up the biuret reagent. The reagent turns from blue to violet in the presence of proteins, blue to pink, when combined with short-chain polypeptides. Take parallel test tubes and put the reagents in them according to the given table. Make up the volume to 5 ml with distilled water (DW). Incubate the first test tubes at 37°C for 10 minutes. Take the first test tube (blank) and set the colorimeter at zero at 540 nm. Measure the O.D of the rest of the left test tube. In Observation Table 6 Protein is 5 mg / ml.
Antioxidant assay
The antioxidant assay was performed using the potassium ferrocyanide reducing method by Oyaizu using Ascorbic Acid (1%) as standard. Aliquots of the extract were taken in a test tube and made up to a volume of 1 ml with distilled water. Then 2.5 ml of Phosphate buffer and 2.5 ml of potassium ferricyanide were added to each of the tubes, and incubation was carried out for 20 min at 50°C in a water bath 2.5 ml of 2.5 ml + Fecl3 = 2.5 ml3. The concentration of the sample was calculated by using the following formula,
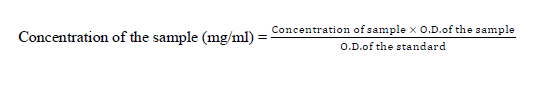
Estimation of gallic acid
We add Folin’s reagent (1 ml) and Sodium bicarbonate (2 ml), and the absorbance was measured at 700 nm in a spectrophotometer.
In the present study, a leaf sample was collected from Noida and 50 % of aqueous, ethanol, and methanol extract were prepared to check the phytochemical. The quantitative analysis of leaf samples of jamun was done to check the presence of phytochemicals. Standard methods were used to determine phenol content, Alkaloids of leaf extracts [34]. There has been a surge in interest in using phytochemicals to treat Trichloro acetic acid was added and centrifuged for 10 min. The supernatant, dist. H2O and FeCl3 were taken in the ratio of 1:1:1.2 and the absorbance was measured at 700 nm in a spectrophotometer. Supernatant means 2.5 ml+Dist. H2O=dementia in recent years [35], and most phytochemicals are extracted from plants [36,37]. Result in the Table 1 showed that the Alkaloids content of selected Syzygium cumini Leaves. Table 2 Estimation of Alkaloids content of selected Syzygium cumini Leaves. Table 3 Total Phenolic content of selected Syzygium cumini Leaves. Figure 1 Reducing sugar standard graph for selected Syzygium cumini Leaves. Table 4 Reducing sugar test observation and standard of selected Syzygium cumini Leaves. Table 5 Estimation of sugar content of selected Syzygium cumini Leaves. Table 6 O.D result of the standard protein content of selected Syzygium cumini Leaves. Table 7 Estimation of protein content of selected Syzygium cumini Leaves. Table 8 Standard of Gallic acid content of selected Syzygium cumini Leaves. Table 9 Standard Table of Antioxidant assay of selected Syzygium cumini Leaves. Table 10 Antioxidant asaay of selected Syzygium cumini Leaves. The alkaloid, phenol, protein, sugar, and antioxidant were present in the leaves of jamun. The results for the dementia in recent years [35], and most phytochemicals are extracted from plants [36,37]. Result in the Table 1 showed that the Alkaloids content of selected Syzygium cumini Leaves. Table 2 Estimation of Alkaloids content of selected Syzygium cumini Leaves. Table 3, Total Phenolic content of selected Syzygium cumini Leaves. Figure 1, Reducing sugar standard graph for selected Syzygium cumini Leaves. Table 4, Reducing sugar test observation and standard of selected Syzygium cumini Leaves. Table 5, Estimation of sugar content of selected Syzygium cumini Leaves. Table 6, O.D result of the standard protein content of selected Syzygium cumini Leaves. Table 7 Estimation of protein content of selected Syzygium cumini Leaves. Table 8, Standard of Gallic acid content of selected Syzygium cumini Leaves. Table 9 Standard Table of Antioxidant assay of selected Syzygium cumini Leaves. Table 10, Antioxidant asaay of selected Syzygium cumini Leaves.The alkaloid, phenol, protein, sugar, and antioxidant were present in the leaves of jamun. The results for the antioxidant assay were found to be between 12 to 20 mg/ml, the highest antioxidant activity was found in 50% ethanol extract. The phenolic content for the sample was found to be between 0.54 to 1.12 mg/ml. The highest value was observed in aqueous extract (50%). The reducing sugar content for the sample was found to be between 1.29 to 2.02 mg/ml. The highest value was observed in ethanolic extract. The alkaloids content was found to be 2.05-6.15 mg/gm, the highest amount was observed in ethanolic extract. The protein content for the leaf sample was found to be between 1.5 and 7.5/ml. The highest value was observed in the case of methanolic (50%) (Tables 1-10) (Figure 1).
| Standard (10% Atropine) (µ l) | OD at 470 nm | Concentration of unknown (mg/ml) |
|---|---|---|
| 0 | 0 | 0 |
| 50 | 0.002 | 0.5 |
| 100 | 0.015 | 1 |
| 150 | 0.022 | 1.5 |
| 200 | 0.039 | 2 |
| Leaf extract | OD at 470 nm | Concentration of sample (mg/ml) |
|---|---|---|
| Aqueous (50%) | 0.04 | 2.051 |
| Ethanol (50%) | 0.12 | 6.153 |
| Methanol (50%) | 0.06 | 3.076 |
| Leaf extract | OD at 700 nm | Concentration of sample (mg/ml) |
|---|---|---|
| Aqueous (50%) | 0.39 | 1.12 |
| Ethanol (50%) | 0.19 | 0.54 |
| Methanol (50%) | 0.22 | 0.63 |
| Standard Maltose | Concentration of Maltose (mg/ml) | DW(ml) | O.D. at 540 nm |
|---|---|---|---|
| BLANK | 0 | 1 | 0 |
| 0.1 | 0.2 | 0.9 | 0.16 |
| 0.2 | 0.4 | 0.8 | 0.32 |
| 0.3 | 0.6 | 0.7 | 0.48 |
| 0.4 | 0.8 | 0.6 | 0.62 |
| 0.5 | 1 | 0.5 | 0.78 |
| 0.6 | 1.2 | 0.4 | 0.94 |
| 0.7 | 1.4 | 0.3 | 1.09 |
| 0.8 | 1.6 | 0.2 | 1.24 |
| 0.9 | 1.8 | 0.1 | 1.39 |
| 1 | 2 | 0 | 1.55 |
| Leaf extract (µ l) | OD at 540 nm | Concentration of sample (mg/ml) |
|---|---|---|
| Blank | 0 | 0 |
| Aqueous (50%) | 1 | 1.29 |
| Ethanol (50%) | 1.56 | 2.02 |
| Methanol (50%) | 1.45 | 1.87 |
| Protein (ml) | Concentration of protein (mg) | Distilled Water (DW) | Biurette reagent | OD at 540 nm |
|---|---|---|---|---|
| 0 | 0 | 2 | 3 | 0 |
| 0.1 | 0.5 | 1.9 | 3 | 0.02 |
| 0.2 | 1 | 1.8 | 3 | 0.05 |
| 0.3 | 1.5 | 1.7 | 3 | 0.07 |
| 0.4 | 2 | 1.6 | 3 | 0.09 |
| 0.5 | 2.5 | 1.5 | 3 | 0.11 |
| 0.6 | 3 | 1.4 | 3 | 0.14 |
| 0.7 | 3.5 | 1.3 | 3 | 0.17 |
| 0.8 | 4 | 1.2 | 3 | 0.19 |
| 0.9 | 4.5 | 1.1 | 3 | 0.22 |
| 1 | 5 | 1 | 3 | 0.24 |
| Leaf extract (µ l) | OD at 540 nm | Concentration of sample (mg/ml) |
|---|---|---|
| Blank | 0 | 0 |
| Aqueous (50%) | 0.31 | 6.4 |
| Ethanol (50%) | 0.07 | 1.5 |
| Methanol (50%) | 0.36 | 7.5 |
| Standard of gallic acid | Dist.H2O in (µ l) | O.D at 700 nm | Concentration of gallic acid (mg/ml) |
|---|---|---|---|
| Blank | 1000 µ l | - | - |
| 10 µ l | 990 µ l | 0.348 | 1 |
| 20 µ l | 980 µ l | 0.394 | 2 |
| 40 µ l | 960 µ l | 0.41 | 4 |
| 60 µ l | 940 µ l | 0.471 | 6 |
| 80 µ l | 920 µ l | 5.72 | 8 |
| 100 µ l | 900 µ l | 6.43 | 10 |
| Standard (0.1% ascorbic acid) | Dist.H2O | O.D. at 700 nm |
|---|---|---|
| (µl) | ||
| Blank | 1000 µl | 1.79 |
| 10 µl | 990 µl | 1.65 |
| 20 µl | 980 µl | 0.656 |
| 40 µl | 960 µl | 1.533 |
| 60 µl | 940 µl | 1.326 |
| 80 µl | 920 µl | 0.722 |
| 100 µl | 900 µl | 0.592 |
| Leaf extract | O.D. at 700 nm | Concentration of sample (mg/ml) |
|---|---|---|
| Blank | 1.498 | - |
| Aqueous (50%) | 1.62 | 12 |
| Ethanol (50%) | 1.502 | 20 |
| Methanol (50%) | 1.543 | 19 |
Jamun has highly beneficial in Ayurvedic medicinal culture it has great health benefits and helps in diabetes cures. Jamun or Jamun powder contains a high amount of protein, fiber, calcium, iron, phosphorus, and several nutrients it also acts as an antioxidant that protects against cancer. A diet high in antioxidants may reduce many diseases including heart disease. Antioxidants protect tissues from damage against reactive oxygen species and other free radicals. Plants contain phytochemicals such as alkaloids, phenol, and essential oils, which have pronounced antimicrobial activity. This underlies the use since antiquity of herbs to improve oral hygiene and prevent common diseases. Herbs or herbal products play a key role in the future of health and hygiene.
In the present study the quantitative analysis of leaf samples of Jamun was done to check the presence of phytochemicals. Standard methods were used to determine phenol content, Alkaloids of leaf extracts. The data demonstrate that the leaves of Jamun include alkaloids, phenols, proteins, sugars, and antioxidants. The antioxidant assay revealed that the 50% ethanol extract had the maximum antioxidant activity. The phenolic concentration of the aqueous extract was found to be the greatest (50%). The ethanolic extract had the highest value in reducing sugar content. The largest concentrations of alkaloids were found in the ethanolic extract. In the case of methanolic, the maximum protein content was reported (50%).Many Herbs products play a significant role in medicine and hygiene. But a fruit Jamun contains more ingredients that have health benefits as it helps in increasing insulin levels and benefits type 2 diabetes patients. Therefore, the government should arrange programs on Jamun trees plantation and aware villager about the health benefits of Jamun.
Ms. Shilpa is thankful to Managing Director, Codon Biotech Pvt. Ltd., Noida, for their encouragement for this research paper. Er. Siddhartha Dan and Mahasweta Mandal are also thankful to Director, for her support.
[Cross Ref] [Google scholar] [Pubmed]
[Cross Ref] [Google scholar] [PubMed]
[Cross Ref] [Google scholar] [PubMed]
[Cross Ref] [Google scholar] [PubMed]