Research Article - Annals of Biological Research ( 2017) Volume 8, Issue 1
In the first stage of our research, C. officinalis and C. arvensis plants having medicinal and economical important were grown from embryo in the MS0 nutrient medium. In the second stage, callus culture was started with leaf explants of C. officinalis and C. arvensis plants and optimized with measuring of biomass in the MS0, MS1, MS3, MS4, MS6 nutrient mediums which were supplemented with combination of different concentrations of auxin and cytokinin. The statistical analysis relating to this measurements of callus biomass was conducted. The most favorable nutrient medium was established to be MS1 (1mg/l NAA+1mg/l BAP) for both C. officinalis and C. arvensis species during four different subculture from the 0. day. The most effective MS nutrient medium was determined to be MS1, MS4, MS3, MS6 and MS0, respectively.
Calendula officinalis, C. arvensis, Callus culture, Auxin, Cytokinin
Calendula officinalis (pot marigold) L., known for its ornamental plant characteristics, is a medicinal plant which is belonging to Asteraceae (Compositae) family. The species grows to 20 up to 40 cm height and has 20 varieties. Its flower appears yellow [1]. Its chemical constituents include triterpene glycosides, triterpene alcohols, flavonol glycosides, essential oil, polysaccharides and fatty oil [2]. Many studies have reported that the plant have pharmacological effects such as anti-cancer [3-6], anti-microbial [7-11], anti-leishmanial [12,13], anti-HIV [14], antioxidants [15-17], cytotoxic, anti-tumor [3,18,19], anti-viral [20], anti-inflamatuar [19,21], oedema diuretic [22], hypoglycemic [23], uterotonic [24], lymphocyte activator effect [3], in venous ulcer treatment [25] and for biligenic function [26].
It is known that the in vitro cultures has some advantages. A few of these advantages is indicated to be the secondary metabolite production, the cell proliferation, etc. under the sterile and controlled conditions in the laboratory during the culture period [27]. The callus was defined the accumulation containing undifferentiated/unorganized parenchymatic cells that was occurred at the injured areas of undifferentiated cells and tissues [28]. It was understood that the secondary metabolites wasn’t produce with callus culture. Additionally, the callus culture was stated that the beginning of cell suspension culture or the transitional culture aimed serial production with the organ formation [29]. The equal ratio of auxin/cytokinin was caused the callus formation [30]. It was explained for in vitro culture that the different biotic (elicitation, etc.) and abiotic (plant growth regulators, metal ions, etc.) stimulating compounds added in the nutrient medium [31-34].
The objective of our study was to evaluate the effect of adding different auxin:cytokinin combinations to enhance callus induction capability of C. officinalis and C. arvensis species from the leaf explants in the callus culture. Otherwise, our study is tought to be the initial step for transition to the cell suspension culture. Therefore, the callus culture and the cell suspension culture would be an important step to obtain the medically and economically important secondary metabolites containing of C. officinalis and C. arvensis species.
Plant materials
It was used that the certificated C. officinalis and C. arvensis seeds were bought from Ceylan Agricultural Company in Turkey.
In vitro culture conditions and sterilization
The plant growth regulators used in the MS nutrient mediums were prepared as stock solution. The different solvents was used for different plant growth regulators. It was used 1N NaOH for NAA (Naphthaleneacetic acid), IAA (Indole acetic acid), 2,4-D (2,4-Dichlorophenoxyacetic acid) and BAP (6-Benzylaminopurine); EtOH (% 99) for 4-CPA (4-Chlorophenoxyacetic acid).
The MS0 medium was prepared by adding 1.1 g/l MS (Murashige and Skoog) (Sigma-S5519) (¼ MS for C. officinalis and C. arvensis species), 15 g/l sucrose (%3) (Sigma-S-5391). In addition to these, the MS nutrient mediums preparing in order to the callus induction was added the various combination of different concentration oxine and cytokinin. The ph of MS medium was adjusted to 5.80 with 1N HCl and 1N NaOH. Subsequently, it was added 8 g/l agar in the MS nutrient medium.
The materials used in the laboratory and the MS mediums were sterilized with 1 atm pressure during 15 min in the autoclave.
Callus culture and statistical analysis
Ten- twelve weeks old leaves of C. officinalis and C. arvensis have been growth in vitro conditions on the MS0 medium for start the calli formation. In order to obtain the calli from explants, MS0 and the twelve different MS medium which supplemented with the different concentrations and combinations of plant growth regulators have been prepeared (Table 1).
| Medium | Auxin (mg/l) | Cytokinin (mg/l) |
|---|---|---|
| NAA (mg/l) | BAP (mg/l) | |
| MS0 | _ | _ |
| MS1 | 1 | 1 |
| MS2 | 1 | 2 |
| MS3 | 0,5 | 5 |
| IAA (mg/l) | BAP (mg/l) | |
| MS4 | 1 | 1 |
| MS5 | 1 | 2 |
| MS6 | 0,5 | 5 |
| 2,4-D (mg/l) | BAP (mg/l) | |
| MS7 | 1 | 1 |
| MS8 | 1 | 2 |
| MS9 | 0,5 | 5 |
| 4-CPA (mg/l) | BAP (mg/l) | |
| MS10 | 1 | 1 |
| MS11 | 1 | 2 |
| MS12 | 0,5 | 5 |
Table 1: The MS nutrient mediums in which the leaf explants were placed and the callus subcultures were transferred (between MS0 and MS12).
Firstly, the leaf explants of C. officinalis and C. arvensis species were incubated on the MS0 and the twelve different MS nutrient mediums during the 30 days. After the 30 days, the amount of callus biomass obtained from the injured areas of leaf explants was weighed on a precision scale and transferred to the fresh MS mediums in the sterile cabine (0. day/at the beginning of callus culture). It was carried out the four subcultures. So, the increased amount of callus biomass was calculated and the callus subculture was completed in 120 days. The maximum amount of callus biomass was obtained from MS1, MS3, MS4 and MS6 nutrient medium in the callus subculture. Furthermore, the MS0 nutrient medium was used to maintain the subcultures because of the control group. The callus subcultures was carried out to be three repetitive, the twenty petri in the each repeat and independently of one another.
The increased amount of callus biomass was compared with the four different callus subculture. Repeated Measurements Variance Analysis ANOVA technique was used to investigate the effect of plant species, subculture and experiments on the callus biomass in the statistically data relating to the callus culture experiments. Tukey Multiple Comparison Test was utilized to determine differences in between plant species, subcultures and experiments compared with the control group (MS0). Statistical analysis was conducted by using Statistica for Windows (Ver. 10) statistics package software.
The results of callus culture
As a result of variance analysis; the together effect of plant species, subculture and experiments (plant species X subculture X experiment) on the amount of callus biomass in compared to the control group was found to be significant statistically (p≤0,005). It was proven that the calli growing on the MS1 nutrient medium (1mg/l NAA+1mg/l BAP) obtained the maximum amount of callus biomass for both two Calendula species and all subcultures.
It was gotten the maximum callus biomass at the end of 4th subculture and this subculture was identified to be the most appropriate subculture and it was confirmed at the end of 4th subculture taken the maximum callus biomass to be the most appropriate subculture in the both of two Calendula species and all of the experiment groups. It was found that the amount of callus biomass was risen with the increasing number of subculture and C. officinalis species had the more higher callus biomass than C. arvensis species in the all subcultures and all of the experiment groups. The maximum amount of callus biomass was acquired from the MS1, MS4, MS3 and MS6, respectively. Furthermore, this nutrient mediums were induced more callus biomass than in the MS0.
As a result of variance analysis; the effect of plant species, subcultures and experiments (plant species X subculture X experiment) on the amount of callus biomass in compared to the control group was found to be significant statistically (p≤0,005). These results were indicated in the Table 2 as the introductory statistics and the results of Tukey Multiple Comparison Test (Table 2).
| Calendula officinalis | Calendula arvensis | |||||||
|---|---|---|---|---|---|---|---|---|
| Experiment | 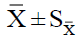 |
Min. | Max. | 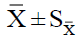 |
Min. | Max. | ||
| Subculture | 0.Day (Beginning) | MS0 | 0.447 ± 0.001 D-e-I | 0.445 | 0.448 | 0.318 ± 0.005 D-e-II | 0.312 | 0.329 |
| MS1 | 1.351 ± 0.006 A-e-I | 1.341 | 1.363 | 0.972 ± 0.001 A-e-II | 0.971 | 0.973 | ||
| MS4 | 1.196 ± 0.020 B-e-I | 1.161 | 1.230 | 0.838 ± 0.006 B-e-II | 0.830 | 0.849 | ||
| MS3 | 1.089 ± 0.002 C-e-I | 1.086 | 1.093 | 0.665 ± 0.002 C-e-II | 0.661 | 0.669 | ||
| MS6 | 1.020 ± 0.003 C-e-I | 1.016 | 1.026 | 0.611 ± 0.003 C-e-II | 0.606 | 0.616 | ||
| I. Subculture | MS0 | 0.556 ± 0.001 E-d-I | 0.553 | 0.557 | 0.394 ± 0.007 E-d-II | 0.383 | 0.406 | |
| MS1 | 2.550 ± 0.006 A-d-I | 2.540 | 2.562 | 1.401 ± 0.001 A-d-II | 1.400 | 1.402 | ||
| MS4 | 2.189 ± 0.019 B-d-I | 2.158 | 2.224 | 1.188 ± 0.006 B-d-II | 1.181 | 1.201 | ||
| MS3 | 1.908 ± 0.002 C-d-I | 1.903 | 1.911 | 0.937 ± 0.002 C-d-II | 0.933 | 0.942 | ||
| MS6 | 1.631 ± 0.004 D-d-I | 1.625 | 1.637 | 0.830 ± 0.003 D-d-II | 0.825 | 0.835 | ||
| II. Subculture | MS0 | 0.665 ± 0.001 E-c-I | 0.663 | 0.667 | 0.464 ± 0.007 E-c-II | 0.453 | 0.477 | |
| MS1 | 3.828 ± 0.013 A-c-I | 3.804 | 3.849 | 1.950 ± 0.001 A-c-II | 1.949 | 1.953 | ||
| MS4 | 3.250 ± 0.019 B-c-I | 3.218 | 3.285 | 1.609 ± 0.005 B-c-II | 1.602 | 1.619 | ||
| MS3 | 2.801 ± 0.006 C-c-I | 2.788 | 2.810 | 1.264 ± 0.003 C-c-II | 1.259 | 1.267 | ||
| MS6 | 2.331 ± 0.004 D-c-I | 2.326 | 2.339 | 1.100 ± 0.002 D-c-II | 1.097 | 1.103 | ||
| III. Subculture | MS0 | 0.776 ± 0.002 E-b-I | 0.772 | 0.779 | 0.528 ± 0.007 E-b-II | 0.517 | 0.541 | |
| MS1 | 5.477 ± 0.044 A-b-I | 5.419 | 5.563 | 2.670 ± 0.001 A-b-II | 2.669 | 2.673 | ||
| MS4 | 4.559 ± 0.018 B-b-I | 4.529 | 4.590 | 2.130 ± 0.011 B-b-II | 2.110 | 2.149 | ||
| MS3 | 3.791 ± 0.006 C-b-I | 3.780 | 3.800 | 1.644 ± 0.001 C-b-II | 1.642 | 1.645 | ||
| MS6 | 3.049 ± 0.002 D-b-I | 3.047 | 3.053 | 1.422 ± 0.002 D-b-II | 1.420 | 1.425 | ||
| IV. Subculture | MS0 | 0.868 ± 0.003 E-a-I | 0.862 | 0.874 | 0.576 ± 0.009 E-a-II | 0.559 | 0.586 | |
| MS1 | 7.673 ± 0.043 A-a-I | 7.609 | 7.755 | 3.535 ± 0.005 A-a-II | 3.527 | 3.544 | ||
| MS4 | 6.171 ± 0.015 B-a-I | 6.144 | 6.195 | 2.732 ± 0.012 B-a-II | 2.711 | 2.752 | ||
| MS3 | 5.013 ± 0.007 C-a-I | 5.000 | 5.025 | 2.089 ± 0.002 C-a-II | 2.086 | 2.093 | ||
| MS6 | 3.897 ± 0.001 D-a-I | 3.895 | 3.900 | 1.794 ± 0.001 D-a-II | 1.793 | 1.795 | ||
Table 2: The statistical information and the results of Tukey Multiple Test with regard to the changing callus biomass.
Note 1: The differences among the mean of experiments showed different capital letters is significant in the same plant species and the same subculture (P≤0.05).
Note 2: The differences among the mean of subcultures showed different small letters is significant in the same plant species and the same experiment (P≤0.05).
Note 3: The differences among the mean of plant species showed different romen numerals is significant in the same subculture and the same experiment (P≤0.05).
The statistical analysis with regard to the increasing of callus biomass between of subcultures, the statistical analysis with regard to the plant species and general introductory statistical analysis was indicated in the Tables 3, Table 4 and Table 5, respectively.
| Culture | 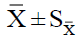 |
Min. | Max. |
|---|---|---|---|
| 0. Day | 0.8505 ± 0.0188 | 0.249 | 3.534 |
| 1. Subculture | 1.3583 ± 0.0307 | 0.314 | 4.7301 |
| 2. Subculture | 1.9261 ± 0.0454 | 0.374 | 6.033 |
| 3. Subculture | 2.6047 ± 0.0645 | 0.438 | 7.753 |
| 4. Subculture | 3.4347 ± 0.0904 | 0.481 | 9.933 |
Table 3: Statistical analysis according to the subcultures.
| Plant Species | 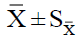 |
Min. | Max. |
|---|---|---|---|
| C. officinalis | 2.7233 ± 0.0504 | 0.3581 | 9.933 |
| C. arvensis | 1.3464 ± 0.0211 | 0.249 | 4.102 |
Table 4: Statistical analysis according to the plant species.
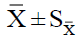 |
Min. | Max. |
|---|---|---|
| 2.0349 ± 0.0301 | 0.249 | 9.933 |
Table 5: General introductory statistical analysis.
In the result of our study, the undifferentiated, light green and white colored fragile callus was obtained from the leaf explants of C. officinalis transferring on the MS nutrient mediums supplemented with the combination of equal concentration of auxin and cytokinin (1:1 mg/l NAA:BAP and 1:1 mg/l IAA:BAP). Furthermore, this MS nutrient mediums were achieved statistically the more callus biomass in comparison with the other MS nutrient mediums tested.
Consistent with our study, [35] was indicated that the most callus biomass (168.5 mg) and the green colored callus was taken from the leaf explants of C. officinalis transferred to the MS nutrient mediums supplemented with the combination of equal concentration of auxin and cytokinin (0.4 mg/l 2,4-D+0.4 mg/l KIN).
In the our research, the undifferentiated, white, light green callus was grown from the leaf explants placed on the MS nutrient medium supplemented with combination of auxin (NAA, IAA) at lower concentration as compared with cytokinin (BAP) concentration (0.5 mg/l NAA+5 mg/l BAP ve 0.5 mg/l IAA+5 mg/l BAP). The efficient callus biomass was also acquired from these nutrient mediums although the amount of callus biomass was obtained lesser than the MS1 and MS4 nutrient mediums. As appropriate with the our research, [36] was stated that the hundred percent of callus formation and the light green callus was taken from the MS nutrient medium supplemented with combination of auxin at lower concentration compared to cytokinin (0.5 mg/l NAA+5 mg/l BAP).
In the our study, it was determined that the amount of callus biomass obtained from the MS nutrient medium supplemented with 1 mg/l NAA+1 mg/l BAP or 0.5 mg/l NAA+5 mg/l BAP was the more than 1 mg/l IAA+1 mg/l BAP and 0.5 mg/l IAA+5 mg/l BAP, respectively. In accordance with the our study, [36] was pointed out that the concentration of NAA have been an important effect on the callus induction from the leaf explants.
In the another research, it was stated that the compact, light colored callus was observed and the 75 percent of callus induction was in the MS nutrient medium supplemented with the combination of IAA (auxin) and Kin (cytokinin). Otherwise, the 100 percent of callus induction was gotten from the MS nutrient medium supplemented with the combination of NAA:BAP [36]. In accordance with this, the amount of callus biomass obtained from in the combination of IAA:BAP was lesser than the combination of NAA:BAP in the our study.
Another researchers was co-cultured the different explants of C. officinalis species on the MS nutrient medium supplemented with 0.5mg/l NAA+2mg/l Kin, 1mg/l NAA+4mg/l Kin and 2 mg/l NAA+6 mg/l Kin. As a result of this, it was stated that the percentage of callus formation was % 85.5, % 73.58 and % 77.5, respectively and the differences among the amounts of this callus formation was not significant. In contrast to our study, it was determined that the highest callus biomass was obtained in a low concentration of NAA (0.5 mg/l) [37]. In the our study, it was established that the maximum amount of callus biomass in the among experimented combinations was obtained from the MS nutrient medium supplemented with NAA:BAP combination used the higher concentration of NAA (1 mg/l).
Two researchers was pointed out that the fluorescence green, friable and fast growing callus was taken from the leaf explants of Pluchea lanceolata (Asteraceae) species grown on the MS nutrient medium supplemented with 2 mg/l NAA+2 mg/l BA. Furthermore, it was emphasized that the best results of fresh and dry callus biomass was gotten from this NAA:BA combination [38]. The results of this research was paralleled with the our research results in terms of fresh callus biomass.
In the another study, it was appeared that % 100 of callus induction was occured in the each MS nutrient mediums supplemented with 0.5mg/l NAA+0.5mg/l BAP, 1mg/l NAA+0.5mg/l BAP, 1.5mg/l NAA+0.5mg/l BAP transferred the leaf explants. So, it was taken the best amount of callus biomass in the combinations of NAA:BAP [39]. Likewise, we were attained the maximum amount of callus biomass as statistically in the combination of NAA:BAP (1:1 mg/l) (MS1) in the result of our study [39] was achieved %100 of callus induction obtained from the MS nutrient medium supplemented with the combination of 2,4-D:BAP transferred leaf explants. In contrast, it wasn’t acquired the enough amount of callus biomass by the combination of 2,4-D:BAP to maintain the callus culture of C. officinalis or C. arvensis species in the our study.
In parallel with our research, [40] was detected that the MS nutrient medium supplemented with the combinations of NAA:BAP transferred the leaf explants of Acmella calva (DC.) R.K.Jansen. (Asteraceae) species was more effective than the combination of IAA:BAP for the purpose of the callus formation.
In accordance with our research, [41] was observed that the addition on the MS nutrient medium of only the 2,4-D or the combination of 2,4-D:NAA:IAA wasn’t a significant effect on the callus induction.
In conclusion of our study, most of the medicinal plants such as C. officinalis and C. arvensis species have very rich secondary metabolites source which are pharmaceutical and economical important. When these plants are cultured as in vitro, it was determined that the formation of callus biomass can be increased by using the different plant growth regulators in most of the cases. Therefore, this callus biomass can be a source of phenolic compounds and carotenoids such as a caffeic acid and a beta carotene will be isolated for pharmaceutical using.
This research is a part of Miss. Nergis KAYA’s Doctorate Thesis in Biological Science which is succesfully completed in Graduate School of Natural and Applied Sciences in Canakkale Onsekiz Mart University under the supervisory of Prof. Dr. Cüneyt AKI. This research project which numbered as FDK 2015-401 was supported by Canakkale Onsekiz Mart University Commission of Scientific Research Projects.