Research Article - Journal of Natural Product and Plant Resources ( 2017) Volume 7, Issue 3
Aloe gilbertii Reynolds, locally known as Eret, is traditionally used for the treatment of several illnesses, including
wounds in Ethiopia. Based on its medicinal claims, the leaf latex of A. gilbertii and its constituents were evaluated for
their potential antioxidant activity using DPPH assay method. Application of the leaf latex of A. gilbertii on silica gel
column chromatography followed by preparative silica gel-TLC led to isolation of three polar anthrones, identified as
microdontin A/B (1), Aloin A/B (2) and Aloinoside A/B (3) by spectral data (IR, 1H, 13C NMR, DEPT and ESI-MS) as
well as by chemical conversion. The leaf latex (IC50=18.2 μg/mL) exhibited a strong free radical scavenging activities
in a dose-dependent manner due to the presence of high concentration of phenolic compounds, such as microdontin
A/B (1), aloin A/B (2) and aloinoside A/B (3) in the leaf latex of A. gilbertii. In light of the above results, the present
study demonstrates that the leaf latex of A. gilbertii and its constitutes can protect the body from oxidative stress from
Reactive Oxygen Species (ROS).
Aloe gilbertii, Aloin A/B, Aloinoside A/B, Microdontin A/B, Antioxidant.
Medicinal plants have played an essential role for treatment of illness throughout human history. Since earliest times, people have gathered these substances to create herbal medicines to treat certain diseases. Today’s plant-based drugs treat a range of diseases, from headaches to cancer [1]. It is estimated by the World Health Organization that up to 80% of people, particularly in developing countries, still rely mainly on traditional remedies of plant origin for their healthcare [2].
The genus Aloe comprises about 600 species, of which 46 are indigenous to Ethiopia and Eriterea [3]. The leaves and roots of aloe species are sources of many interesting secondary metabolites which belong to different classes of compounds, including anthraquinones, pre-anthraquinones, anthrones, bianthraquinoids, chromones, flavonoids, coumarins and pyrones [4,5]. Anthraquinone are used as laxative and also in the treatment of fungal skin diseases [6].
A. gilbertii is one of the endemic Aloe species in Ethiopia grouped together with other succulent shrub aloes such as A. calidophila and A. megalacantha in the south and eastern Ethiopia, respectively. It is also mainly characterized by erect, ascending or sprawling stems and distinguished from related species by the cylindrical to sub-clavate perianth [7]. A. gilbertii leaves are traditionally used for treatment for several illnesses in Ethiopia [8].
As part of our continuing efforts [9,10] in the search for potential biologically active compounds from Ethiopian Aloes, we have isolated and characterized three polar compounds from leaf latex of A. gilbertii. These isolates, along with the leaf latex of A. gilbertii, were evaluated for their potential antioxidant activity using DPPH assay method.
General
IR spectra were carried out in KBr plates on a Perkin-Elmer BX (400-4000 cm-1) instrument. NMR spectra were recorded on a Bruker Avance DMX400 FT-NMR spectrometer operating at 400 MHz for 1H and 100 MHz for 13C at room temperature. Signals were referred to an internal standard TMS. Chemical shifts were reported in δ units and coupling constants (J) in Hz. ESI-mass spectra were recorded on an Ultimate 3000 LC-MS with negative mode. The source voltage and temperature were fixed at 3 kV and 250°C. 1-diphenyl-2-picrylhydrazl (DPPH) (Sigma). CC: silica gel 60 F (230 ± 400 mesh, Merck). TLC: silica gel 60 F (Merck). Prep. TLC: silica gel 60 F self made plates (Merck); spots and bands were viewed by UV light (254 and 366 nm). TLC and prep. TLC solvent system (CHCl3:MeOH; 4:1).
Plant material
The latex of A. gilbertii was collected in October 2011 from Jello Kebele, Shashemene Woreda, Arsi Zone, some 250 km south of Addis Ababa, Ethiopia. The plant was authenticated by Professor Sebsebe Demissew, the National Herbarium, Department of Biology (DoB), Addis Ababa University (AAU) where voucher specimen was deposited with collection number AG 01.
Leaf latex preparation
The leaf latex of A. gilbertii was collected by cutting the leaves near the base and allowing the yellow sap to come down in a plate. The latex was then left in open air for three days to allow evaporation of water, which yielded a yellow powder.
Isolation of compounds
The leaf latex was initially fractionated by column chromatography on silica gel eluting with EtOAc-MeOH gradients, followed by prep. TLC using system (CHCl3:MeOH; 4:1), resulting in the isolation of three major compounds 1, 2 and 3, with Rf value of 0.55, 0.35 and 0.16, respectively as shown in Figure 1.
Antioxidant activity testing
Free radical scavenging activities of the latex and isolated compounds were determined by DPPH assay method as described by Cuendet (1997) [11] where 50 μL of various known concentrations of the test samples was mixed with 5 mL of 0.004% methanol solution of DPPH. The mixture was incubated for 30 min at 37°C.
After incubation, the absorbance of the mixture was read at 517 nm using UV spectrophotometer (Jenway Model 6500, England). Tests were carried out in triplicate and average values were taken. Inhibition of DPPH radical was calculated using the equation:
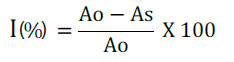
Where Ao is the absorbance of the control (containing all reagents except the test compound), and As is the absorbance of the tested sample. The IC50 value which represented the concentration of the samples that caused 50% inhibition was determined for all test samples.
Column chromatography of the leaf latex of A. gilbertii followed by a preparative silica gel-TLC resulted in isolation of three anthrones, identified as microdontin A/B (1), Aloin A/B (2) and Aloinoside A/B (3) with Rf value of 0.55, 0.35 and 0.16, respectively as shown in Figure 1.
Structural elucidation of the isolated compounds
Compound 1: Compound 1 was obtained as a pale yellow amorphous substance with Rf value of 0.55 (CHCl3:MeOH; 4:1). Compound 1 gave a pseudomolecular ion at m/z of 563 in negative-mode ESI-mass spectrum, corresponding to a relative molecular weight of 564 mu. A molecular formula of C30H28O11 was deduced based on ESI-MS, 1H and 13C NMR data.
The IR spectrum of compound 1 displayed the presence of hydroxyl (3415 cm-1), ester carbonyl (1710 cm-1), carbonyl (1635 cm-1) and aromatic groups (1603 cm-1). 1H and 13C NMR spectral data of compound 1 showed a number of signals well above the number of protons and carbons in the molecular formula. However, a close analysis showed some of the signals occurred in pairs, indicating compound 1 is a mixture of two closely related compounds. From the data presented in IR, 1H-NMR, 13CNMR, DEPT, ESI-MS and by comparing 1H and 13C NMR data of compound 1 with a similar compound reported from other Aloe microdonta [12], the structure of compound 1 was deduced as mixture of microdontin A and B, as shown the structure in Figure 2.
Compound 2: Compound 2 was also isolated from the leaf latex as a yellow amorphous solid with Rf value of 0.35 (CHCl3:MeOH; 4:1). A molecular formula of C21H22O9 was deduced for compound 2 by negative-mode ESI-mass spectrum (m/z=417 [M-H]-), 1H and 13C NMR data. The IR spectrum indicated the presence of hydroxyl (3394 cm-1), carbonyl (1635 cm-1) and aromatic (1618 cm-1) functional groups.
As indicated below in Compounds Data section, the 1H and 13C NMR spectral data of compound 2 were similar to that of compound 1, except the absence of coumaroyl moiety in 2. In addition, acid hydrolysis of compound 1 afforded compound 2 (co-TLC). Therefore, as depicted in Figure 2, compound 2 was identified as a known aloin A/B (10-C-β- D-glucopyranosyl-1, 8-dihydroxymethyl-9-anthracenone) by comparing its 1H and 13C NMR data with the same compound reported from Aloe excelsa [13].
Compound 3: Compound 3 was isolated as a yellow amorphous solid from the leaf latex of A. gilbertii with Rf value of 0.16 (CHCl3:MeOH; 4:1). ESI-mass spectrum of compound 3 showed a pseudomolecular ion at m/z 563 in the negative mode, corresponding to a relative molecular weight of 564 mu. This along with 1H and 13C NMR data gave a molecular formula C27H32O13 for compound 3.
Compound 3 exhibited a pattern of signals in the 1H and 13C NMR spectra similar to that of compound 2, except for the presence of additional signals due to a rhamnose moiety. The presence of an O-rhamnose unit in compound 3 was confirmed by conversion of compound 3 to 1 on acidic hydrolysis. Complete assignments of protons and carbons are listed below in Compounds Data section. From the spectroscopic data obtained for compound 3 and comparison of the NMR chemical shifts those reported in the literature [14,15], compound 3 was identified as 10-glucopyranosyl-1, 8-dihydroxy-3-(rhamnopyranosyl-hydroxymethyl)-9(10H) -anthracenone, commonly called aloinoside A/B (3).
Acid hydrolysis of 1 and 3: A solution of compound 1 (10 mg) in 2% methanolic HC1 (3 mL) was stirred for 6 hours at room temperature. After removal of the solvent, the reaction mixture was neutralized with 10% NaHCO3 and extracted with EtOAc to give 5 mg of 2 (co-TLC and 1H NMR). Likewise, acid hydrolysis was carried out with compound 3 to afford compound 2 (co-TLC and 1H NMR).
Compounds data: Microdontin A/B (1): A pale yellow amorphous substance; Rf=0.55 (CHCl3:MeOH; 4:1); IR cm-1: 3415, 1710, 1635, 1603; -Ve ESI-MS m/z: 563 [M-H]-, indicating a relative molecular weight (Mr) of 564 (C30H28O11); 1H NMR δ ppm: 12.01/12.09 (2H, s, 1-OH), 11.88/11.94 (2H, s, 8-OH), 6.77/6.92 (2H, d, H-2), 7.16/7.25 (2H, brs, H-4), 7.10/7.26 (2H, d, H-5). 7.58/7.60 (2H, d, H-6), 6.71/6.90 (2H, brs, H-7), 4.53/4.57 ( 2H, d, H-10), 4.60 (4H, s, H2-15), 2.95-4.30 (14H, m, C-Glucose), 7.35 (4H, d, p-coumaroyl H-2” and H-6”), 6.82 (4H, d, p-coumaroyl H-3” and H-5”), 7.44/7.46 (2H, d, p-coumaroyl H-7”), 5.84/5.95 (2H, d, p-coumaroyl H-8”), 10.06 (2H, brs, p-coumaroyl 4”-OH); 13C NMR δ ppm: 160.12/160.17 (C-1), 112.81/113.30 (C-2), 152.41/152.67 (C-3), 117.97/118.46 (C-4), 120.45/121.11 (C-5), 136.39 (C-6), 115.98/116.22 (C-7), 161.92/162.00 (C-8), 193.26/193.36 (C-9), 45.03/45.28 (C-10), 142.67/143.00 (C-11), 115.38/116.18 (C-12), 115.38/116.18 (C-13), 142.67/143.00 (C-14), 62.83/62.91 (C-15), 61.77-83.73 (C-Glucose), 125.67 (p-coumaroyl C-1”), 129.15/130.57 (p-coumaroyl C-2”),116.35 (p-coumaroyl C-3”),162.06/162.40 (p-coumaroyl C-4”),116.35 (p-coumaroyl C-5”),132.06/130.62 (p-coumaroyl C-6”),144.61/144.81 (p-coumaroyl C-7”), 114.34/114.42 (p-coumaroyl C-8”),167.43 (p-coumaroyl C-9”).
Aloin A/B (2): A pale yellow amorphous substance; Rf=0.35 (CHCl3:MeOH; 4:1); IR cm-1: 3394, 1635, 1618; -Ve ESIMS m/z: 417 [M-H]-, indicating a relative molecular weight (Mr) of 418 (C21H22O9); 1H NMR δ ppm: 11.85/11.89 (2H, brs, 1-OH), 11.79/11.80 (2H, brs, 8-OH), 6.83/ 6.85 (2H, brs, H-2), 7.01/ 7.02 (2H, d, H-4), 7.06/ 7.08 (2H, dd, H-5), 7.54/ 7.55 (2H, t, H-6), 6.88/ 6.89 (2H, dd, H-7), 4.57 (2H, s, H-10), 4.81/4.91 (4H, s, H2-15), 2.72-3.99 (C-Glucose); 13C NMR δ ppm: 161.21/161.38 (C-1), 112.77/113.10 (C-2), 151.80/152.60 (C-3), 116.67/118.26 (C-4), 119.36/120.7 (C-5), 136.5/135.68 (C-6), 115.85/116.20 (C-7), 161.58 (C-8), 193.83 (C-9), 44.31/44.61 (C-10), 146.08/146.30 (C- 11), 117.49/117.81(C-12), 116.26 (C-13), 142.25/142.47 62.82/62.85 (C-15), (C-14), 60.27-85.60 (C-Glucose).
Aloinoside A/B (3): A pale yellow amorphous substance; Rf=0.16 (CHCl3:MeOH; 4:1); IR cm-1: 3400, 1618, 1600; -Ve ESI-MS m/z: 563 [M-H]-, indicating a relative molecular weight (Mr) of 564 (C27H32O13); 1H NMR δ ppm: 11.84/11.85 (2H, brs, 1-OH), 11.79/11.80 (2H, brs, 8-OH), 6.84/6.85 (2H, brs, H-2), 7.00/ 7.05 (2H, brs, H-4), 7.07/ 7.08 (2H, d, H-5), 7.55/ 7.58 (2H, t, H-6), 6.88/ 6.91 (2H, d, H-7), 4.53/ 4.60 (2H, brs, H-10), 5.05/5.08 (2H, s, H2-15), 2.70-4.82 (C-Glucose), 0.95-5.25 (O-Rhamnose); 13C NMR δ ppm: 161.24/161.33 (C-1), 113.93/114.10 (C-2), 146.93/147.69 (C-3), 115.90/116.22 (C-4), 119.36/120.72 (C-5), 135.80/136.68 (C-6), 117.63/119.14 (C-7), 161.43 (C-8), 193.80 (C-9), 44.45/44.51 (C-10), 146.04/146.35 (C-11), 116.10/116.71 (C-12), 116.80/117.51 (C-13), 142.27/142.57 (C- 14), 61.82 (C-15), 61.79-85.46 (C-Glucose), 18.42-100.29 (O-Rhamnose).
Free radical scavenging activity
The DPPH method is one of the many antioxidant models, widely used as a reliable tool to measure free radical scavenging activity of plant extracts and their constituents [16]. The results depicted in Table 1 demonstrated the strong ability of the latex of A. gilbertii to act as an electron or hydrogen atom donor in a dose-dependent manner with a ranging of 26.5-64.0 % for the tested concentrations of 12.5-100 μg/mL. The IC50 of the latex in this assay was found to be 18.2 μg/mL, while that of ascorbic acid was 4.6 μg/mL. This free radical scavenging effect of the leaf latex of A. gilbertii was comparable with other species of Aloe from Ethiopia [9,17].
| Test substances | IC50 values (µg/mL) |
|---|---|
| Leaf latex | 18.2 |
| Microdontin A/B (1) | 14.3 |
| Aloin A/B (2) | 56.7 |
| Aloinoside A/B (3) | 83.5 |
| Ascorbic acid | 4.6 |
Note: All experiments were performed in triplicate and average values were taken.
Table 1: The IC50 values of the leaf latex of Aloe gilbertii and its constituents in DPPH assay in comparison with ascorbic acid (reference sample).
All the isolated compounds exhibited a free radical scavenging property in a dose-dependent manner, of which microdontin A/B (1) (IC50=14.3 μg/mL) was found with the highest activity. This might be due to the presence of additional phenolic group (p-coumaroyl unit) as the part of the structure in microdontin A/B. Aloinoside A/B (3), on the other hand, showed relatively weaker activity (IC50=83.5 μg/mL). The observed reduction of DPPH by the latex and the isolated compounds was either due to the transfer of a hydrogen atom or the transfer of an electron. Since phenols are very important plant constituents because of their scavenging ability due to their hydroxyl groups and may contribute directly to antioxidative action [18]. Therefore, strong free radical scavenging activity of the latex may be due to the presence of high concentration of phenolic compounds, such as microdontin A/B (1), aloin A/B (2) and aloinoside A/B (3) in the leaf latex of A. gilbertii. Antioxidant potential displayed by the leaf latex of A. gilbertii might help to reduce the oxidative stress which further prevents the occurrence of other diseases.
In the present study, three anthrones, microdontin A/B (1), aloin A/B (2) and aloinoside A/B (3), were isolated and identified from the leaf latex of A. gilbertii. It is interesting to note that all three constituents were found to have free radical scavenging activities due to the presence of phenolic groups. The highest free radicals scavenging activities was recorded for microdontin A/B because of the presence of additional phenolic group (p-coumaroyl unit) as the part of the structure in microdontin A/B. This, together with the obtained results, suggests that the free radical scavenging activity of the latex and its constituents increases as the dose increases. In light of the above results, the present study demonstrates that the leaf latex of A. gilbertii and its constitutes can protect the body from oxidative stress from Reactive Oxygen Species (ROS).
Supplementary data: IR, ESI-MS, 1H, 13C, DEPT-135 NMR spectra data for all compounds are included in the supplementary data.
The authors are grateful to Prof. Sebsebe Demissew, the National Herbarium, Addis Ababa University for identification of the plant material. Abayneh Kassahun would like to acknowledge the Department of Chemistry, Debre berhan University for sponsoring this study.