Research Article - Journal of Natural Product and Plant Resources ( 2017) Volume 7, Issue 3
Natural indicators with the pigments extracted from natural sources like plants, animals, minerals, etc. have been grown in the past decades due to its potential applicability over a wide range of applications and the availability of the resources. Although, these natural dyes have been used for centuries, its durability is considered as the main drawback for their applicability. However, they are eco-friendly, biodegradable, and non-carcinogenic in comparison to synthetic pigments. In this research work, the pigment was extracted from Hibiscus rosa-sinensis, i.e., shoe-flower and the extract was used as an indicator over a wide range of acid-base titrations.
Hibiscus, Natural indicators, pH, Pigments, Titration.
In this century, a global awareness is already in place for favoring the utilization of the vast diversity of natural resources for protecting the environment and earth from pollution and ecological imbalances. In this scenario, the natural dyes have been obtained from various sources [1-3], such as plants [4-6], insects [7], animals [8], and minerals [7]. Flower, fruit, bark, leaf, seed, any other parts of the plants come under the class of plant based natural dyes [4-6], whereas, Cochineal and lac are the insects used to extract the dyes [7]. Mollusk, murex snail, cuttlefish, and shellfish are the animals used for the natural dye extraction [9]. In addition, the minerals that include, clay, ochre, and malachite are utilized for the natural dye production [7]. Although there are several sources for the natural dyes, many natural dyestuff and stains were obtained mainly from plants [5, 6, 9]. These colour pigments are popular for their use in food materials, pharmaceuticals, and textiles, in place of their synthetic counterparts.
In addition, the dyes especially from the natural resources can also be applied as indicators, which are less harmful to the environment. Flower and leaf pigments often fit in this description [1]. In particular, the extracts or juice from rose petals and red cabbage are well known examples for the acid-base indicator solution [10, 11]. Anthocyanin, a flavonoid pigment, is the most readily available acid-base indicator, commonly extracted from plants and its parts [12-17], and they mainly found in vegetables, including purple cabbage, beets, blueberries, cherries, raspberries, and purple grapes [18]. Within the plant they serve as key antioxidants and pigments contributing to the colouration of flowers. Moreover, anthocyanins are water soluble strong colours and have been used to colour food from since historical eras. Likewise, alizarin, curcumin, esculin, and logwood are the other acid-base indicators that are extracted from plants [19, 20]. An acid-base indicator is commonly a weak acid with differently coloured acid and conjugate base forms explained in the equation (01). In addition, the equilibrium constant for this dissociation and the pH expression can be explained by the equations (02-05).

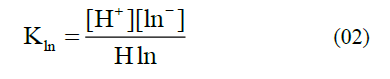
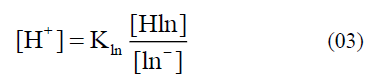
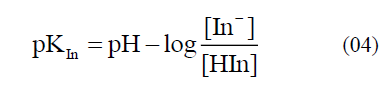

The position of the equilibrium changes such that HIn is converted to In- while the pH changes from acidic to basic. This conversion results in a decrease in [HIn] and a corresponding increase in [In-].
The acid-base or pH indicator is a chemical detector of hydronium ions (H3O+) or hydrogen ions (H+). The pH indicator changes its colour depend upon their proton accepting and donating ability. However, the unavailability of the conventional indicator for the weak-acid weak-base titration is the main drawback, and, thus, there is a need to find the suitable indicators to do the analytical quantification using titrimetry.
In this respect, this study investigates the natural indicator extracted from the shoe-flower (Hibiscus rosa-sinensis) for a wide range of acid-base titrations. The shoe-flower tree is a bushy, evergreen or small tree grows mainly in tropical and sub-tropical part. Although there are some existing literatures talk about of the extraction of Hibiscus family flowers and plants [21-23], the study on this Hibiscus rosa-sinensis towards the analytical purpose is still lacking, and, thus, this study is expected to deliver the knowledge on the anthocyanin extract from the shoe-flower, and its applications towards acid-base titrations. The anthocyanin pigment in the flower petals influences in determining the endpoint by using the colour changes during the acid base titration, and our results indicate that this indicator can be used as weak acid-weak base indicators in the acid-base titration, where there are no conventional indicators to perform this quantification.
Collection of plant materials
Hibiscus rosa-sinensis flower petals were collected from Jaffna peninsula, Sri Lanka in the months of March to June, 2016.
Preparation of natural indicator
14.0 g of fresh shoe-flower petals were taken and cut into small pieces. 30 mL of distilled water was added into the beaker contain the small pieces of flower petals. The plant sap was boiled at 60°C for 10 min. with vigorous shaking. Finally, the supernatant extract was filtered through the suction pump. The collected anthocyanin of Hibiscus rosa-sinensis solution was stored in a refrigerator at 4°C.
Preparation of Buffer solution
Buffer solutions for 14 different pH values were prepared and the accurate pH of the buffer solutions was tested with the pH meter.
Spectroscopic analysis
The solutions with different pH were prepares by adding 3 drops of the shoe-flower extract into the prepared buffer solutions, and the absorbance of the shoe-flower with different pH were recorded in the visible region by using UVVIS- NIR Spectrophotometer (JASCO V 570).
Titration
Four different types of acid-base titration, such as strong acid vs. strong base (HCl vs.NaOH), strong acid vs. weak base (HCl vs. NaOH ), weak acid vs. strong base (NH4OH vs.CH3COOH), and weak acid vs. weak base (CH3COOHvs.NH4OH) with the acid-base concentrations of 1.0 mol L-1 and 0.1 were carried out with the natural indicator (shoe-flower extract) separately, and the results was compared with the results those were obtained from the titration with the conventional indicators, methyl orange and phenolphthalein.
The hot extraction of the shoe-flower petals in the presence of water resulted a violet-red colour dye solution as illustrated in Figure 1(A). The anthocyanin, cyanidin-3-sophoroside which is available in the Hibiscus rosa-sinensis [24], as shown in Figure 1(B) may found to be the reason for this observed colour.
Spectroscopic analysis
The absorption curve of red shoe-flower extract shows an absorbance maximum at 520 nm, whereas the absorbance maxima shifted to different wavelengths due to the series of colour changes with pH, when the pH of the solution changes and the colour observed at different pH is illustrated in Figure 2.
In addition, the colour changes observed with the shoe-flower extract has a good agreement with the prescribed colours of anthocyanin in the itemized reagents. These results indicate the presence of anthocyanin in the petals of red shoe-flower, and their colour changes with different pH. The absorbance vs. wavelength peak is illustrated in Figure 3.
In the acidic pH, such as pH<4, the absorbance maxima were observed at 520 nm, whereas a red-shift was attained with the pH>4. The peak maximum was recorded at 529.5 nm, 537.5 nm and with the pH 4, 5, and 6, respectively. The solutions at pH 8, 9, and 10 show further red shift and the absorbance maxima was recorded at 560.0 nm, 549.5 nm and 568 nm. The solution at pH 11 and 12 show the peak maxima at around 580 nm, however, the intensity seems to decrease with the solution of pH 12. In particular, no peak maxima were observed in the visible region for the extract at pH 13 and 14, indicate that there was a lost in the conjugation of the anthocyanin chromophore. These results indicate that the hibiscus extract act as a potential indicator over a range of pH.
Furthermore, it can be seen clearly from the absorbance spectra of the natural indicator (Figure 3) that the maximum absorbance wavelength (λmax) of the solution incessantly changes from one to another pH due to the changes of chromophore in the anthocyanin presence in the natural indicator. However, the selected conventional indicator illustrated in Figure 4, shows a sharp change in λmax from one to another colour during the changes in the solution pH from acid to base and vice versa.
The equilibrium constant of the indicator explained in the equation (02) cannot be calculated for the natural indicator (Hibiscus rosa-sinensis) used in this study due to the continuous changes of λmax value with a function of pH (i.e., absence of isobestic point).
Titration
Four different types of acid-base titration, such as strong acid vs. strong base (HCl vs. NaOH), strong acid vs. weak base (HCl vs. NH4OH), weak acid vs. strong base (NH4OH vs. CH3COOH), and weak acid vs. weak base (CH3COOH vs. NH4OH) were examined.
The titration was also carried out with the standard phenolphthalein and methyl orange indicator for comparison purpose. The average titre volume determined from 3-5 different titrations in each set and the colour changes and the required volume of 1.0 mol L-1 titrant are listed in Table 1.
| Titrand | Titrant | Required volume of titrant (mL) | Colour change |
|---|---|---|---|
| NaOH | HCl | 10.2 | Yellow to Pale pink |
| NH4OH | HCl | 8.7 | Greenish yellow to Pale pink |
| HCl | NaOH | 9.75 | Dark pink to Blue |
| CH3COOH | NaOH | 10.5 | Pale pink to Pale yellow |
| NaOH | CH3COOH | 9.5 | Yellow to Colourless |
| HCl | NH4OH | 9.9 | Dark pink to Blue |
| NH4OH | CH3COOH | 4.2 | Greenish yellow to pale pink |
| CH3COOH | NH4OH | 19.6 | Pale pink to Pale yellow |
Table 1: The required volume of 1.0 mol L-1 titrant and the colour changes observed during the titration when shoe-flower extract was used as indicator.
The colour of the indicator changes with pH, and the average titre volumes were determined for each type of acid base titration by considering three to five different individual readings. These values found to have a good agreement with the values obtained with the conventional indicators, and, thus, the natural indicator employed in these acid base titrations was economic, safe, and an efficient alternative for traditional indicators. Furthermore, chemical indicators are more expensive and hazardous in nature and, thus, lead to affect the environmental sustainability.
In comparison to the conventional indicators, such as phenolphthalein, methyl orange, etc., the natural indicators are very cheap, easy to extract, and eco-friendly. Therefore, these natural indicators could be excellent replacement materials for conventional indicators. Moreover, it is noteworthy to mention here that these natural indicators work properly even at a low concentration (0.1 mol L-1) of all type of acid base titrations, except the weak acid and weak base titration due to the very less intense of their colour changes observed during these weak acid and weak base titrations. The colour change observed and the required volume is tabulated in Table 2.
| Titrand | Titrant | Required volume of titrant (mL) | Colour change |
|---|---|---|---|
| NaOH | HCl | 10.35 | Yellow to Pale pink |
| NH4OH | HCl | 9.6 | Greenish yellow to Pale pink |
| HCl | NaOH | 9.6 | Dark pink to Blue |
| CH3COOH | NaOH | 10.1 | Pale pink to Pale yellow |
| NaOH | CH3COOH | 10 | Yellow to Colourless |
| HCl | NH4OH | 10.3 | Dark pink to Blue |
| NH4OH | CH3COOH | N/A | Greenish yellow to pale pink |
| CH3COOH | NH4OH | N/A | Pale pink to Pale yellow |
| N/A refer not applicable | |||
Table 2: The required volume of 0.1 titrant and the colour changes observed during the titration when shoe-flower extract was used as indicator.
The observed colour change for the titrations NH4OH vs CH3COOH and CH3COOH vs. NH4OH is unclear, and it result difficulties in finding the end points. Therefore, these results indicate that the low concentration ranges are not possible for the usage of natural indicators. However, the changes was very significant at the higher concentration level of 1.0 mol L-1 in which the conventional indicator fails to show the colour changes for these weak base and weak acid titrations.
The results obtained in this study specify the potential use of natural indicators over a wide range of acid-base titration. The colour changes observed in this study was due to the presence of anthocyanin, and a very sharp colour changes occurred at the end point of all the different titrations due to the changes in the chromophore of the anthocyanin present in Hibiscus. In addition, the similar reading of required volume obtained with the standard indicator; further validate the applicability of these natural indicators for these titrations. Moreover, the natural indicators are always beneficial due to its eco-friendly nature, ease of preparation, and the applicability.
The authors would like to express their sincere thanks to the Department of Chemistry, University of Jaffna, Sri Lanka for their support to complete this research work successfully.