Research Article - Annals of Biological Research ( 2017) Volume 8, Issue 3
Chemical relaxers or hair straighteners are used to manage hair coarseness and straightness which can pose health risks and possible toxic effects to humans. Hence, this study targeted in assessing and evaluating embryo-toxic and teratogenic affects of hair relaxers on developing zebrafish embryos. The Fish Embryo Toxicity (FET) test was utilized to determine the toxicity and teratogenicity of zebrafish embryos. Concentrations of hair relaxers used were T1=0% (Control), T2=100%, T3=50%, T4=30%, T5=10%, T6=5%, T7=4%, T8=3%, T9=1%, T10=0.5% and T11=0.05%. Percent mortality and percent hatchability were recorded after 12 and 24 h post-treatment application (hpta). Statistical analyses showed significant differences on the percent mortality and percent hatchability between the treatments. Treatment concentrations ranging from 3%-100% were documented as lethal to zebrafish embryos while teratogenic effects were evident on the control, 0.05%, 0.5% and 1% treatment concentrations, respectively. These can be attributed to the chemical compositions and very basic pH of the hair relaxer. Therefore, this study was able to show the presence of teratogenic and embryo-toxic effects of chemical hair relaxers to developing embryos, which were not previously reported.
Chemical hair relaxers, Embryo-toxic, Teratogenic, Zebrafish, FET
Personal care products (PCPs) or cosmetic products, including hair care products (e.g. shampoos, conditioners, surfactants, mineral oils, hair dyes and relaxers) have been widely used by consumers in a daily basis [1]. Hair care products are mostly used with the purpose or intention of not only grooming and maintaining hair strength but also to modify its appearance to the preference of the user [2]. As such, hair color, coarseness or straightness can be modified with the use of these products.
Chemical hair relaxers were developed in the late 1940’s and have been mixed later on to utilize calcium hydroxide, sulfites, creams, sodium hydroxide (lye), lithium hydroxide and ammonium thioglycolate [2-4]. Such chemical relaxers react with chemical bonds that result in the straightening or flattening of coarse and wavy hair [4]. However, chemical relaxers are known to have irritating effects to the scalp, such as skin burns because of the high pH levels causing burns and scalp lesions [4]. There are also recorded cases of ingestion of chemical relaxers [5] which resulted to esophageal and stomach damage (ulceration) [6]. Chemical relaxers are also known to have ingredients that are hormonally active (causing disruptions of estrogen and progesterone) [3]. The presence of these compounds in relaxers also link perming or hair straightening as a potential cause of abnormalities in the reproductive system [3,7].
This study aims to evaluate the teratogenic and embryo-toxic activities of selected hair relaxer against zebrafish (D. rerio) embryos. The findings of this study also provide additional information on the adverse effects of chemical hair relaxers using zebra fish embryos as the model organism. There has been a speculated correlation between the exposures to chemical hair relaxers with maternal-related diseases such as prenatal abnormalities. Women (especially pregnant or lactating), can be more knowledgeable of the hazards, if ever or effects of using hair straightening products, and with such, this study has been conducted. This study was only limited to the evaluation of the embryotoxic and teratogenic effects of a chemical hair relaxer of a known brand in the Philippines.
Acquisition of test substance
A commercially available hair relaxer cream of a known brand in the Philippines was acquired from a local shop. The chemical relaxer chemical composition includes aqua, paraffinum liquidum, cetyl alcohol, cetyl ammonium chloride, stearic acid, glycerin, triethanolamine, calcium pantothenate, tocopheryl acetate (Vitamin E), lactic acid, aloe barbadensis (Aloe Vera) extract, fragrance, methyl paraben and Disodium EDTA. The pH level of the chemical hair relaxer was obtained using pH meter.
Preparation of embryo water
The preparation of the embryo water was done following the formulation of Hank’s solution for teratogenic assays [8]. The working solution (embryo water) was a combination of 1 mL of Hank’s solution 1 (prepared by combining 8 g of Sodium chloride (NaCl), 0.4 g of Potassium chloride (KCl) and 100 mL of double distilled water); 0.1 mL of Hank’s solution 2 (prepared by adding 0.67 g of Na2HPO4 • 7 H2O and 0.60 g of KH2PO4) and 100 mL of double distilled water); 1 mL of Hank’s solution 4 (prepared by a combination of 1.92 g of CaCl2 • 2 H2O and 100 mL of double distilled water); 1 mL of Hank’s solution 5 (prepared using 2.46 g of MgSO4 • 7 H2O and 100 mL of double distilled water); 0.35 g of NaHCO3 and 96.5 mL of double distilled water. 1 M of NaOH was gradually added to the solution until a working solution was brought to the pH of 7.2. The pH of the solution was checked using the pH meter.
Preparation of chemical hair relaxer concentration
Spawning of zebrafish embryos
Twelve mature zebrafishes were obtained commercially with 2:1 ratio of male-to-female fishes. The zebrafish pairs were housed separately in fish tanks with clean and untreated tap water. The fish tank was covered by a black sheet to initiate breeding and spawning of zebrafish for 12 h [9]. Zebrafish eggs, at approximately 12 h post-fertilization (hpf) [10], were then collected from the spawn trap after 30 min of subjection to artificial light [11]. Fertilized embryos were selected for abnormalities or fatalities, which can be determined by the transparency of each embryo [9].
Fish embryo toxicity (FET) test
Fertilized zebrafish embryos were placed on 24-well multi-well plates containing embryo water [11]. The multiwell plates will be treated with 4 mL of the test chemical concentrations (Table 1) in each well. The multi-well plates with zebrafish embryos were incubated under 26 ± 1°C. Zebrafish embryos were monitored and observed in 12, 24, 36, 72 h post-treatment application (hpta) under a microscope to assess morphological endpoints and toxicity of the chemical hair relaxer.
| Treatment | Percent Concentration (%) | Chemical Hair relaxer (mL) | Embryo water (mL) |
|---|---|---|---|
| T1 | 0 | 0 | 10 |
| T2 | 100 | 10 | 0 |
| T3 | 50 | 5 | 5 |
| T4 | 30 | 3 | 7 |
| T5 | 10 | 1 | 9 |
| T6 | 5 | 0.5 | 9.5 |
| T7 | 4 | 0.4 | 9.6 |
| T7 | 3 | 0.3 | 9.7 |
| T8 | 2 | 0.2 | 9.8 |
| T9 | 1 | 0.1 | 9.9 |
| T10 | 0.5 | 0.05 | 9.95 |
| T11 | 0.05 | 0.005 | 9.995 |
Table 1: Preparation of the different concentrations of chemical hair relaxer with the corresponding concentrations of the embryo water in specific percent concentration.
Presentation of data and evaluation of morphological endpoints, hatchability & mortality rate of Zebrafishes
The data gathered in the study were used to assess the a) morphological endpoints of zebra fish embryos, b) percent hatchability, c) percent mortality. Morphological endpoints and abnormalities from zebra fish embryos were based on the endpoints identified by the OECD guideline on fish embryo acute toxicity test [10]. The percent hatchability will be based from the ratio of number of hatched zebra fish eggs over the number of initial embryos after posttreatment incubation.
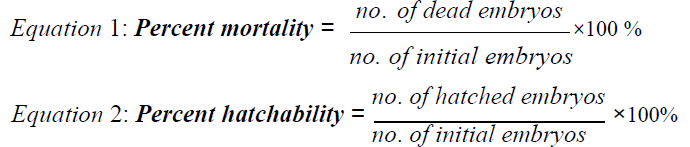
Statistical analyses
SPSS software version 20.1 program were used to analyze the data collected. All gathered data were laid out in a completely randomized design. To check which treatments are significantly different from each other, the data were subjected to One-Way Analysis of Variance, Duncan Multiple Range Test and Tukey’s Test.
The zebrafish embryos were observed against the different concentrations of a commercial brand of a hair relaxer at 12 and 24 h post-treatment application (hpta) and were assessed for lethality and teratogenicity. Zebrafish embryos exposed to treatment concentrations ranging from T2 to T8 (3% to 100%) were recorded to have 100% mortality rate and 0% hatchability rate. Meanwhile, the morphological assessment for the treatments with higher concentrations reflected coagulation of the embryos (i.e., observed in T2, T3, T4, T5 and T6). Formation of key morphological features such as the head, tail and yolk were seen on treatments T7, T8 and T9. At T9, 2 out of 3 zebrafish embryos were considered to be dead after 24 hpta.
Hair relaxer concentrations of 0.5% (T10) and 0.05% (T11) were treated on zebrafish embryos and were observed at 12 and 24 hpta. For 0.5% (T10) hair relaxer concentration, the embryo has developed and hatched at 12 hpta although the embryo died prior to microscopic observation. Various abnormalities and even absence of important morphological features such as somites were evident on zebrafish embryos exposed to T10 after 24 hpta (Figure 1). For T11, there is no recorded morphological abnormality and the appearance and morphological/physiological features of the embryo tested is almost identical to that of the control treatment.
Figure 1: : Lethal and teratogenic effects of chemical hair relaxers against zebrafish embryos after 24 hpta. (A) Coagulation of zebrafish embryo after exposure to T2 (100%); (B) Developmental delay and teratogenicity as reflected on the malformation of head and tail regions exhibited by zebrafish embryos exposed to T6 (5%); (C) Ruptures on yolk sac and head regions of the zebrafish embryos exposed to T10 (0.50%); and (D) Zebrafish with normal development.
Co: Coagulated; mt: malformed tail region; mh: malformed head region; ysr: yolk sac ruptured; rh: ruptured head region.
For the recorded abnormalities among treated zebrafish embryos at 24 hpta and recorded embryo hatching. It was also observed that there was 90% mortality rate and only 10% hatchability rate on the embryos exposed to the chemical hair relaxer solutions. Percent mortality and percent hatchability of zebrafish embryos have significant differences among treatment concentrations. Based from the pH concentrations of the treatment groups obtained, T2 (100%) and T3 (50%) has a pH level of 10, while T4-T11 (30%-0.05%) have pH level of 7.5.
For the Fish Embryo Toxicity test, the treated zebrafish embryos were observed at 24 hpta and a very low survival rate and low hatching rate among tested embryos was recorded. For the treatments T2-T8, all the tested embryos died after 24 hpta. It was also observed that the severity of developmental defects in the embryos were evident as the concentration of the chemical relaxer increases. Treatments with the highest hair relaxer concentrations have coagulated embryos (dead) after 24 hpta while embryos exposed to treatments with lower concentrations have developed, but with visible abnormal morphological features.
Statistical analyses revealed that zebrafish embryos exposed to treatments 3%-100% (T2-T8) have lethal or toxic effects leading to the death of the embryos. On the other hand, zebrafish embryos exposed to treatments 0.05%-1% (T9-T11) exhibited abnormal morphological features. Hence, it can be observed that chemical hair relaxer concentrations higher than 1% is already lethal to the zebrafish embryos, while those exposed to hair relaxer concentrations of 0.05%-1% exhibited teratogenic which resulted to abnormalities in their features. However, prolonged exposure of zebrafish embryos to chemical hair relaxer concentration (24 hpta) caused immediate death of embryos.
Active components such as alkaline relaxers ethanolamine thioglycolate and ammonia were present in the product tested. The hair relaxer cream itself is basic at pH 9.5 because of the presence of such alkaline straighteners. These alkaline straighteners play a role in straightening hair. The high pH of the substance is necessary to cause hair emulsion and opening of hair cuticles, having the alkaline agent react with keratin in hair to break disulfide bridges, causing curled hair to soften and straighten [4]. Hair relaxers and other hair product components have potential teratogenic risks, including ethanolamine, although in very high levels of intoxication [12]. In addition, ethanolamine thioglycolate was identified to cause dermal irritation on test animals and other thioglycolate salts identified to have genotoxic and reproductive toxic activities [12]. Another identified component that has identified toxic properties is methyl paraben (p-hydroxybenzoic acid ester derivative). Parabens can penetrate through human skin without being broken down by esterases [13]. In addition, parabens are known to have cytotoxic, genotoxic and oestrogenic properties [14,15], however, they do not possess teratogenic properties [16]. Another potential toxic component in the hair relaxer formulation is disodium EDTA (ethylenediamine tetraacetic acid). EDTA and its salts are known to be cytotoxic and weakly genotoxic chelating agents, and oral exposure to test animals produced reproductive toxicity and developmental delay in tested animals [17]. In addition, an aerosolized formulation of the EDTA salt produced reproductive toxicity through inhalation [17]. The above-stated components of the hair relaxer cream may contribute to the low survivability, low hatching rate and morphological abnormalities exhibited by the tested embryos after exposure to chemical hair relaxer.
Another possible explanation of the low survival rate among zebrafish embryos is the pH of the hair relaxer or its components. The pH level of the control group (embryo water) was 7.2; on the other hand, the pH level of 100% and 50% treatment concentrations were too basic (10.00) while for the T4-T11, the pH is 7.5. The difference in the pH levels of the treatment concentrations and the control group can be considered as one of the factors for the survival rate of the treated zebrafish embryos. Studies have been reported that the maximum pH tolerated by the zebrafish embryos is 8.5 ± 1.5 [7,10]. However, the range of most hair relaxer/rebonding creams is about 9-14, mainly due to the presence of alkaline straighteners such as ethanolamine glycolate and ammonia [4]. This suggests that treatment concentrations higher than 1% may have been too concentrated, turning the environment alkaline which zebrafishes cannot tolerate. On contrary, the improvement of the survival rate of zebrafish embryos at concentrations lower than 1% was evident.
Zebrafish embryos exposed to different concentrations of chemical hair relaxer was proven to have embryotoxic (lethal) and teratogenic effects. It was observed that the concentrations of chemical hair relaxer higher than 1% caused lethality to zebrafish embryos after 24 hpta. On the other hand, at concentrations ranging from 0.05% to 1% resulted to abnormalities to the growth of zebrafish embryos, however, prolonged exposure of the embryos also led to death. The embryotoxicity and teratogenicity of chemical hair relaxer can be attributed to the components present in the test substance as well as the pH level of the solution.
The authors would like to express their gratitude to the Department of Science and Technology, the Philippine Science High School-Central Luzon Campus especially the faculty of Chemistry and Life Sciences Unit for providing assistance and guidance in the completion of this study; and lastly, the authors would like to thank the reviewers of this article for their helpful comments and suggestions.