Research Article - Journal of Natural Product and Plant Resources ( 2017) Volume 7, Issue 4
Angiogenesis is one of the important hallmarks of cancer that include both cancer progression and metastasis.
The recent advances in natural product research stread light on identification and isolation of non-toxic antiangiogenic
and antitumor compound from natural source. In current study, our team made an attempt to evaluate
the anti-angiogenic activity of various crude extracts of Moringa oleifera Lam leaves. The extracts were prepared
by successive soxhlation and the anti-angiogenic activity was evaluated by employing in vivo CAM assay model.
The test extracts were administered at 1, 10 & 100 μg doses and percentage inhibition of neovascularization was
observed after 48 h. Among all the extracts, ethyl acetate extract of Moringa oleifera Lam at a dose of 100 μg showed
significant inhibition of neovascularization (70%) when compared with other extracts.
Antiangiogenic activity, Moringa oleifera. L, Ethyl acetate extract, CAM assay
In 1935, Hertig coined the term angiogenesis later Folkman revealed the mechanism of tumor angiogenesis [1]. The growth of new vessels from existing vasculature is called as angiogenesis predominantly occurring in embryogenesis later, in various physiological (reproductive cycle) and pathological conditions (wound healing) and it is a tightly regulated process by maintaining a balance between angiogenesis factors and angiogenic inhibitors. The new blood vessels growing excessively as in case of cancer, and metabolic disorders like diabetic, etc., or insufficiently as in case of severe wounds and ischaemic heart disease (IHD). Therefore, targeting angiogenesis has been an important therapeutic approach for the treatment for these diseases [2,3].
Tumor growth requires an increment of vascular growth. Therefore, it is angiogenesis-dependent. Tumors lacking angiogenesis remain dormant indefinitely and rapid logarithmic growth follows the acquisition of blood supply. Tumor angiogenic switch seems to be activated when the balance of angiogenic inhibitors to stimulators is shifted toward a proangiogenic milieu shifted toward a proangiogenic milieu. Therefore targeting and modulating antiangiogenic pathways and developing antiangiogenic drug for therapeutic purposes is of great interest in recent years [4].
Since antiquity, Natural products or their derivatives have been used for the treatment of many ailments. The use of herbal drugs as combination therapy has been suggested by different researchers to inhibit the angiogenesis in patients with solid tumors. Among these, attention toward the Moringa oleifera.L has been increased in the recent years. In Traditionally system of medicine this plant has wide applications that includes treatment renal disorders, ulcer, tumor, diabetes etc [5,6].
Moringa oleifera. Lam is a small native tree of the sub-Himalayan regions of North West India, which is now indigenous to many regions in Africa, Arabia, South East Asia, the Pacific and Caribbean Islands and South America. There is evidence that the crude extract of the leaves exhibited significant anticancer activity. However, there is no significant evidence of antiangiogenic property of this plant. Therefore, the present study was undertaken to explore the antiangiogenic property of M. oleifera leaves by employing Chick chorioallantoic membrane (CAM) assay methodology
CAM assay has been widely used to study angiogenesis. It is performed in fertilized chicken eggs, which was first developed by Dr. Joseph Leighton, is one of the conventional histological procedures [7] associated with the proliferation of new vessels and tumor neovascularization by direct observation, where the number of blood vessels was counted [8,9].
Chemicals
All solvents used in the current study (n-hexane, chloroform, ethyl acetate, methanol) were of analytical grade and procured from M/s. Sigma Aldrich, India.
Plant material
The Leaves of Moringa oleifera L. used in the present study was collected from natural habitat in and around Nellore, Andhra Pradesh. The plant is authenticated by Prof. P. Jayaraman M. Pharm., Ph.D., Director, Plant Anatomy Research Centre, Medicinal Plant Research unit, Tambaram, Chennai, (Regd. PARC/2015/3020). A voucher specimen was deposited at institutional herbarium for future reference.
Extraction
The plant material obtained were dried in hot air oven (30-35 0 C) for a period of 5 days. The dried leaves were processed and reduced to fine powder (# 40 Size mesh) mechanically. About 160 g of pulverized plant material was subjected for successive soxhlet extraction as previously described procedure [10]. The extraction was carried out using 650ml of each solvent including n-hexane, chloroform, ethyl acetate, methanol (99.8%) respectively for 14 h and the obtained extract was concentrated to dryness under reduced pressure using rotary vacuum evaporator (Buchi-R200) and stored at 4 0 C until used.
Phytochemical Analysis
Phytochemical analysis was carried out with all the obtained extracts using conventional protocol like alkaloids, flavonoids, carbohydrates, glycosides, saponins, proteins, phenols, fixed oils, mucilage etc.
Test for Alkaloids
The extract was treated with diluted HCl and filtered. The filtrate was treated with Mayer’s reagent, appearance of cream color indicates presence of alkaloids.
Test for flavonoids (Shindoa’s test)
The extracts were dissolved in alcohol, to which a piece of magnesium followed by drop wise addition of Conc. HCL was added and heated. Appearance of magenta color indicates presence of flavonoids.
Test for Carbohydrates
The extracts were treated with 3 ml of α- naphthol in alcohol and Conc. Sulphuric acid were carefully added to side of the test tubes. Formation of a violet ring at the junction of two liquids indicates presence of carbohydrates
Test for Steroids
The extracts were treated with Conc. Sulphuric acid and glacial acetic acid followed by acetic anhydride, a violet ring appears at the junction of the liquids and appearance of green color in the aqueous layer indicates presence of steroids.
Test for Proteins
To the extracts copper sulphate solution followed by sodium hydroxide solution were added. A violet color precipitates indicates presence of proteins.
Test for Phenols
The extracts were treated with neutral ferric chloride solution, appearance of violet color indicates presence of phenols.
Test for Gums and Mucilage
The extracts were treated with 25 ml absolute alcohol and then the solution was filtered. The filtrate was examined for swelling properties.
Test for Glycosides
A pinch of the extract was dissolved in glacial acetic acid and few drops of ferric chloride solution was added followed by the addition of Conc. Sulphuric acid, formation of red ring at the junction of the two liquids indicates presence of glycosides.
Test for Saponins
1ml of the extract was diluted to 20 ml with distilled water, formation of about 1cm foam in the upper part of the test tubes indicates the presence of saponins.
Sample preparation
Stock solution (1mg/ml) of each extract was prepared by using 5% dimethyl sulfoxide (DMSO, v/v). From this the working solutions of varying concentrations were prepared by serial dilutions and the working solutions were filtered using 0.2μm sterile syringe filters. The pH of each solution was maintained between 6.5 and 7.5.
Preparation of Filter Paper Disc
A filter paper disc of size 5 mm was prepared by using a 2-holed puncher to form the paper. Later the prepared disc was sterilized by autoclaving for 15 minutes.
Chick Chorioallantoic Membrane Assay (CAM assay)
CAM assay was carried out for all the extracts according to the assay procedures described previously [11]. A 5 days old fertilized chicken eggs were obtained from a local hatchery, ooty, Tamilnadu. About 5ml of albumin was withdrawn by using a sterile syringe and eggs are allowed for incubation for a period of 6h to allow the CAM detachment from the outer shell. The test extracts were dissolved in DMSO (5%v/v) and loaded in to sterile paper disc at varying concentrations (1, 10, 100μg). sterile paper discs loaded with the vehicle were used as negative control. A group 0f 6 eggs were assigned for each concentration. Later Discs are applied on the CAM through a small window (1×1cm) made on the shell. Later, window was closed with the help of and a sterile surgical tape and eggs were allowed for incubation for a period of 48 h. The images of each egg treated CAM were captured and analyzed for blood vessels in the disc application site and the percentage inhibition was calculated [12,13].
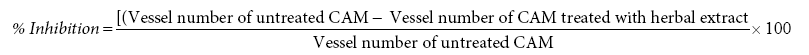
Visual Assessment and Photography
In this assay, the CAM was examined after an incubation period of 48 quantitation involves counting the number of CAM vessels around the area of filter paper disk [14]. In general proangiogenic stimuli, leads the newly formed blood vessels to appear like a disk in a wheel-spoke pattern. In contrast inhibition of angiogenic stimuli leads to lack of formation of new blood vessel. For the assessment of CAM four quadrants in the area were drawn. The branch point of each blood vessel was counted manually in a clockwise direction in each area of the quadrant.
Statistical analysis
The results obtained were analyzed by One-way ANOVA to evaluate the significant difference of means among various treatment groups using Graphpad prism 6.0 software. The values are presented as mean ± S.D.
Extraction
The yield of various extracts of Moringa oleifera leaves are given in Table. 1. Among the extracts, methanol extract was obtained at highest yield (20 % w/w) followed by n-hexane, chloroform and ethyl acetate extracts respectively.
| S. No. | Solvent | Yield of extract (g) | % yield |
|---|---|---|---|
| 1 | n-Hexane | 7 | 4.3% |
| 2 | Chloroform | 5 | 3.12% |
| 3 | Ethyl acetate | 4 | 2.5% |
| 4 | Methanol | 32 | 20% |
Table 1: Percentage yield of M.oleifera leaves
In vivo CAM assay
Antiangiogenic effect of various test extracts investigated by CAM assay suggested that among all the extracts ethyl acetate extract showed significant antiangiogenic activity with % inhibition of 70, 60 % at concentrations of 100 and 10 μg respectively, however a moderate activity was observed with methnol extract at 100 μg. The obtained results were presented in Table 2 and Figure 1
| Extracts | No. of vessels in untreated CAM | No. of vessels in treated CAM | % Inhibition | ||||
|---|---|---|---|---|---|---|---|
| Conc. (µg) | Conc. (µg) | ||||||
| 1 | 10 | 100 | 1 | 10 | 100 | ||
| n-hexane | 9 ± 1.31 | 8 ± 1.22 | 7 ± 2.76 | 7 ± 2.97 | 11.15 | 18.01 | 22.22 |
| Chloroform | 11 ± 2.48 | 9 ± 2.14 | 10 ± 2.18 | 8 ± 1.78 | 18.12 | 9.09 | 27.26 |
| Ethyl acetate | 10 ± 3.12 | 9 ± 1.13 | 4 ± 1.43 | 3 ± 3.22 | 10.01 | 60.00 | 70.00 |
| Methanol | 13 ± 1.66 | 10 ± 2.23 | 9 ± 2.90 | 8 ± 3.14 | 23.07 | 30.76 | 52.21 |
Values are Mean ±SD
Table 2: Antiangiogenic activity of M.oleifera leaf extract
The use of folk medicine in treatment of various diseases were known from ages. And it is well supported with the recent scientific findings. One among the well renowned medicinal plant is Moringa oleifera.L. that is being widely used as edible plant[15]. However, scientific studies that demonstrate the medicinal properties of these plant were significantly less. In order to rationalize the traditional claims regarding its antiangiogenic potential are rather scanty. Therefore determining the antiangiogenic potential of Moringa oleifera.L which would eventually stread light on new leads from this plant[16].
In the present study Antiangiogenic activity of crude extracts of [17] were tested through in vivo CAM model. among all the extracts ethyl acetate extract showed significant antiangiogenic activity with % inhibition of 70, 60 % at concentrations of 100 and 10 μg respectively, however a moderate activity was observed with methnol extract at 100 μg. the results obtained were represented in Table 2 and Figure 1. The medicinal and pharmacological actions of medicinal herbs are often depended to the presence of bioactive compounds called secondary herbal metabolites The qualitative phytochemical study results revealed the presence of steroids and phenolic compounds in ethyl acetate extract. Many natural compounds belonging to this class have been reported to possess cytotoxic and anticancer potentials [18,19]. Therefore, the preliminary results obtained in the present study serves researchers round the globe to evaluate further in order to isolate a potent antiangiogenic agent from this plant.
The result obtained from the present study suggest that M.oleifera.L leaf extract has a potent antiangiogenic property which is evident from the reduce in average branch points in the CAM significantly dose-dependent manner. From the above results it is evident that ethyl acetate extract of M.oleifera.L leaves has a promising antiangiogenic agent and could be a possible source of chemotherapeutic agent in the treatment of tumors. However, further study is required in order to evaluate the mechanisms involved by which M.oleifera.L leaf extract inhibits vascularization in the CAM as well as the pathological relevance of these findings.
The authors were grateful to Department of Science & Technology (DST)- INSPIRE, New-Delhi, India (Grant No. DST/INSPIRE/03/2014/001953) for financial assistance.