Review Article - Der Pharmacia Lettre ( 2023) Volume 15, Issue 7
Received: 01-Mar-2022, Manuscript No. DPL-23-84342;
Editor assigned: 03-Mar-2022, Pre QC No. DPL-23-84342 (PQ);
Reviewed: 17-Mar-2022, QC No. DPL-23-84342;
Revised: 21-Apr-2023, Manuscript No. DPL-23-84342 (R);
Published:
28-Apr-2023
, DOI: 10.37532/0975-5071.2023.15.01
, Citations: Sahu, et al. 2023. A Systemic Review on Recent Outbreaks of Dengue and Newer US FDA Approved Drug. Der
Pharma Lett.15:01-05
,
Copyright: © 2023 Vinayak V. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
This review is an update of dengue recent outbreaks and newer US FDA approved drugs based on worldwide data. Dengue is a self-limited, universal viral infection transmitted among person to person by mosquitoes. Mostly cases are asymptomatic or mild and can be managed without complications. It is estimated that 390 million dengue virus infections are reported per year of which 96 million is observed clinically with many severe symptoms as per WHO and CDC. The pathophysiology of dengue fever (DENV) is not properly understood. Primary indication of disease includes capillary leak syndrome (plasma leakage due to dengue hemorrhagic fever specific dysfunction of endothelial cells), thrombocytopenia (seen in all type of DENV infection), hemorrhagic tendencies and leukopenia. Furthermore clinical dengue fever can be diagnosed by NS1 antigen capture test, NS1 antigen capture test, antibody test and serological method as well as cross reactive Flaviviruses. Whatsmore literature surveys were suggested that medical procedure using the techniques and formulation i.e. nasogastric intubation, intramuscular injections and arterial punctures as well as aspirin, ibuprofen, or other Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) as well as other coagulants however lack of efficacy, The U.S. food and drug administration has approved the first vaccine for the management of dengue in May, 2019 which is known as Dengvaxia proper name dengue tetravalent vaccine, live manufactured by Sanofi Pasteur Inc. Furthermore used for the management of dengue caused by any serotype of dengue virus (DENV-1, DENV-2, DENV-3, and DENV-4) and is for the people who have laboratory confirmed dengue infection and between the age of 9 to 16.
Dengue, Dengue virus, NSAID’S, Tetravalent vaccine, Leukopenia.
Dengue fever is also known as break bone fever or dandy fever. It is a mosquito borne viral disease which is caused by the Dengue Virus (DENV). Dengue virus is spread through the biting of female mosquitos mainly of the species Aedes aegypti and family Flaviviridae. The mosquito becomes infected when it takes the blood of a person who is infected with the virus [1]. After generally bite during the daytime and are found everywhere and are found to be at the peak of their activeness at dawn and dusk. There are four different serotypes of the virus that cause dengue (DENV-1, DENV-2, DENV-3, and DENV-4) [2]. It is mainly found in tropical and sub-tropical climates of our planet, to a large extent in urban and semi-urban areas [3].
Mild dengue fever causes a high fever and flu like symptoms. Severe dengue fever also known as haemorrhagic fever, can cause serious bleeding, a sudden drop in blood pressure (shock) and death [4]. Dengue has an unexpected attack and these symptoms could be an indicator of its onset; high fever, muscle and joint pain, vomiting, skin rashes, pain behind eyes, headache, bleeding from mouth and nose [5]. It is usually a self-limited illness [6]. There is no specific treatment for dengue although proper observation of patient on time, identifying signs and symptoms and suitable management of case accompanied by supportive care with analgesics, fluid replacement and sufficient bed rest can lower the mortality rates of severe dengue to below 1% and prevent patient death [7]. If symptoms progress to severe dengue fever, then medical care is required to reduce the threat of death. Medication which can be used in the treatment of dengue fever is acetaminophen (paracetamol) [8]. Aspirin, other salicylates, and Non-steroidal Anti-Inflammatory Drugs (NSAIDs) should be avoided as they increased risk of bleeding [9]. And clinical approaches can be Nucleic Acid Amplification Test (NAATs), NS1 antigen capture test, antibody test, serological test and cross reactive Flaviviruses [10,11].
Literature surveys were revealed that overall information on recent outbreaks of dengue virus in Bangladesh, Nepal and Pakistan. Additionally the newer drug approved by U.S. food and drug administration along with its mode of transmission, pathophysiology, symptoms, diagnosis and management.
Outbreak of dengue
Dengue is the most rapidly spreading mosquito borne viral disease in the world [12]. The breakout of dengue has grown dangerously around the world in recent decades [13]. Mostly cases are asymptomatic or mild and can be managed without complications [14]. It is estimated that 390 million dengue virus infections are reported per year of which 96 million is observed clinically with many severe symptoms [15]. According to world health organization, the cases have increased over 8 fold over the last two decades, from 505,430 confirmed cases in 2000, to over 2.4 million confirmed cases in 2010, and 5.2 million confirmed cases in 2019 [16]. And it is observed that mostly younger age group is affected [17]. In 2020 and 2021, the cases were seemed to be decreased but suddenly in 2022 January to 28 September in Nepal a total of 28,109 cases were confirmed among them 38 confirmed deaths [18]. Between 1 January and 27 September 2022, a total of 25,932 cases were confirmed in Pakistan and 62 deaths were confirmed [19]. And recently, in Bangladesh since 1 January to 20 November 2022, a total of 52,807 cases were confirmed and 230 confirmed deaths have been reported by the ministry of health and family welfare of Bangladesh.
Mode of transmission
Pathogenesis of dengue is mostly due to mosquito bite, perinatal transmission, blood transfusion, organ transplantation, needle stick injury or laboratory accident. Dengue fever is a most common form of viral infection that is carried out and spread by the mosquitos. Categorically, female mosquitos mainly of the species Aedes aegypti and family Flaviviridae become a vector or carrier of the virus when it bites an infected human. This infected mosquito goes on to bite another human, and the cycle continues. After virus incubation for eight to ten days, an infected mosquito is capable to penetrate and transmit the virus for the rest of its life. The mosquito picks up the virus as part of its blood meal when it feeds on human blood. Only the female mosquitos’ bites and feeds on blood, which she needs to mature her eggs. This virus stays in the mosquito’s gut for up to ten days, after which the insect is capable to spread the infection to any other human it bites and feeds on. In this way, infected humans act as a viral source, with the mosquito picking up the infection from humans and spreading it to uninfected individuals. The virus spreads in the bloodstream of humans for a period of two to seven days, and then the mosquito may acquire the virus when it bites the infected individual. During this time, symptoms of the infection such as fever begin to appear on infected individual. The mosquito’s mostly feeds during the day time. These mosquitos of the species Aedes aegypti and family Flaviviridae breeds nearer to the water and lays their eggs in the walls of the water containers. Therefore, insects are mainly found near the water bodies, unsealed septic tank, water wells, decorative fountains, discarded tires, boats, and bottles including all the other vehicles that are used in the storage of water. There are total four different types of the strains of dengue virus (DENV-1, DENV-2, DENV-3, DENV-4) and infection with single strain ensure life long protection against that strain only. Thus, it is possible for an individual to be infected with rest of three strains.
Pathophysiology
Dengue fever is one of the most rapidly expanding diseases of the tropical and sub-tropical areas, with over two billion people at risk of infection and millions of cases are reported every year. The severe form of the disease, dengue haemorrhagic fever is a most significant cause of hospitalization and death among children in the south-east Asia, western pacific and Americas region. The pathophysiology of dengue fever (DENV) is not properly understood. Primary indication of disease includes capillary leak syndrome (plasma leakage due to dengue haemorrhagic fever specific dysfunction of endothelial cells), thrombocytopenia (seen in all type of DENV infection), haemorrhagic tendencies, and leukopenia. It is known that the major viral envelope of glycoprotein in the virus helps to bind the host cells, followed by viral replication. As the data suggests, monocytes are primary target. Infected monocytes induce the production of Interferon-a (IFN-a) and (IFN-b). Envelope, precursor membrane protein, and Non-Structural protein 1 (NS1) are the most major DENV proteins which are targeted by antibodies as part of the host immune response. According to studies, DENV specific CD4+, helper T cell and CD8+, cytotoxic cells that induce apoptosis in cells. T lymphocytes attacks the infected cells of an individual and releases (IFN-g), Tumour Necrosis Factor-a (TNF-a), and lymphotoxin. This primary infection induces a lifetime immunity of the individual to that specific serotype (DENV-1, DENV-2, DENV-3, and DENV-4), but not to the secondary infection of that individual by another serotype among all the four serotypes. Dengue begins suddenly after a regular incubation period of five to seven days, and this follows three phases: Febrile, critical, and recovery. The pathophysiology can be well defined and understandable with the help of flowchart based on how the healthy individual acquires the dengue virus from the vector or carrier.
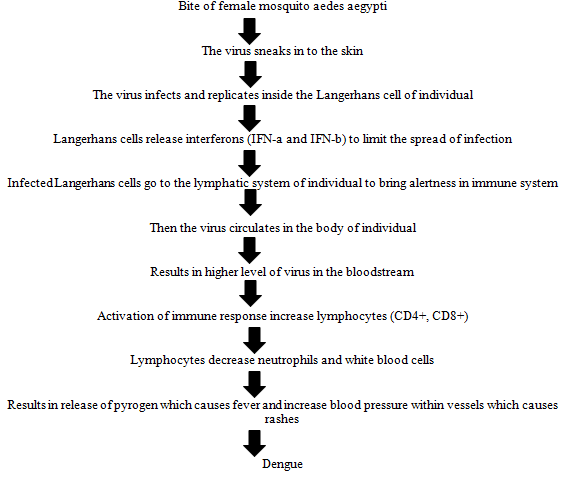
Symptoms
Data’s were compiled and literature surveys were suggested that Infection with any of the four different serotypes of dengue virus (DENV-1, DENV-2, DENV-3, and DENV-4) be able to multiple range of symptoms from mild to severe life threatening haemorrhagic fever and shock. Many infected individuals can experience no signs and symptoms of a dengue infection.
Symptoms of dengue can be classified as mild and severe; the mild symptoms of dengue usually begin after four to ten days followed by biting of an infected mosquito one can be confused with other illness such as flu which causes fever, aches, pain and skin rashes. Dengue fever causes a high fever about 40 degrees celsius, headache, muscle or joint pain, nausea, vomiting, pain behind the eyes, swollen glands, and skin rashes. Most people recover with these sign and symptoms within a week whereas in some cases the situation gets worsen and become life threatening and, in this case, the severe symptoms are seen which is also known as dengue haemorrhagic fever or dengue shock syndrome. This happens when your blood vessels become damaged and the number of platelets in your bloodstream drops down which can lead to shock, internal bleeding, organ failure and even death. Warning sign and symptoms of severe dengue fever usually begins after the first or second day following when fever goes away. Severe dengue fever causes an acute stomach pain, persistent vomiting, bleeding from your gums or nose, observation of blood in your urine, stools or vomit, bleeding under the skin, which might look like bruising, difficult or rapid breathing, fatigue, irritability, or restlessness. Severe dengue fever is a life threatening medical emergency for an individual. The severe form of dengue is primarily seen in individuals involved in a secondary infection with a different serotype. Immediate medical consultation is required if an individual is having sign and symptoms of severe dengue fever. The three phases of dengue include febrile, critical, and recovery. During the febrile phase, an immediate high fever of about 40 degrees celsius takes place which usually lasts for two to seven days. In the critical phase, fever of about 37.5 degrees Celsius to 38 degrees celsius takes place which usually lasts for one to two days. The recovery phase entails the slow reabsorption of extravascular fluid in two to three days.
Diagnosis
Dengue fever may be dangerous depending upon the situation and stage of a patient. All patients with clinically suspected dengue should receive proper management to reduce the risk of complications resulting from increased vascular permeability, organ failure, and plasma leakage. Various tests can be performed clinically:
• Nucleic Acid Amplification Test (NAATs): This test should be performed after less than seven days following onsetof the symptom, serum specimen is collected and detected by viral genomic sequences with rRT-PCR or dengue Non-Structural protein 1 (NS1) antigen by immunoassay. Presence of virus by rRt-PCR or (NS1) antigen in a singlespecimen is confirmation of dengue in patient.
• NS1 antigen capture test: The Non-Structural viral protein (NS1) is an absolute diagnostic target as it is excreted frominfected cells of an individual and is found to be circulating in the blood. It is detected from the onset of symptoms tonine days or more. NS1 can be detected as viral RNA.
• Antibody test: These tests are primarily used to detect two different classes of antibodies produced by the body inresponse to the dengue fever in an individual, these antibodies are IGM and IGG. In which IGM antibodies areproduced first after seven to ten days. After a few months, IGM antibodies gradually fall below and IGG antibodies areproduced more slowly to a response of dengue fever infection. Then, the level rises with an acute infection for longtime.
• Serological method: serological methods such as Enzyme Linked Immunosorbent Assays (ELISA), may confirm thepresence of a recent or past infection, with the detection of anti-dengue antibodies. IgM antibody is detectable after oneweek of infection whereas, IgG antibody takes time to develop and remains in the body for years. The existence of IgMantibody indicates recent DENV infection and existence of IgG antibody indicates of a past infection.
• Cross reactive Flaviviruses: If infection has occurred in a site where other potentially cross reaction Flaviviruses circulate, then both the molecular and serologic diagnostic testing for dengue should be performed. People who areinfected with other Flaviviruses may build cross reactive Flavivirus antibodies, resulting in false positive serologicdengue tests.
Management
So far, there is no specific treatment or an anti-viral medication for dengue is available. So, basically treatment of dengue is dependent on the patient’s illness phase or severity of the symptoms. Those who have mild symptoms can be treated without complications based on being hydrated by drinking plenty of water and adequate oral fluids, getting proper bed rest, and treating muscle and joint pain with acetaminophen. Consumption of ibuprofen, aspirin, other salicylates and Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) should be avoided as they increased risk of internal bleeding. Those who have severe symptoms and warning signs possess complications in treatment, should admit in hospital as soon as possible for proper treatment under the guidance of professional doctor. They can be initiated on intravenous crystalloids, and the fluid rate is titrated based on the patient’s response, stabilizing vital signs, and normalizing haematocrit. Blood transfusion is justified in case of severe bleeding or suspected bleeding when the patient remains unstable, and hematocrit falls despite adequate fluid resuscitation. Platelet transfusion is performed when platelet count drops to less than twenty thousand cells/microliter and these is more risk of bleeding. Invasive medical procedures such as nasogastric intubation, intramuscular injections, and arterial punctures as well as aspirin, ibuprofen, or other Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) and other coagulants as they increased risk of high bleeding thus, should be avoided. Intravenous fluids are excreted during the last and recovery phase to avoid fluid accumulation. When fluid accumulation occurs and vital signs are stable, the administration of more fluid should be avoided to remove excess fluid. If the patient has passed the critical phase, a diuretic loop is used to remove excess fluid from circulation. The US food and drug administration has approved the first vaccine for the management of dengue in May, 2019 which is known as Dengvaxia proper name dengue tetravalent vaccine, live manufactured by Sanofi Pasteur Inc. Furthermore used for the management of dengue caused by any serotype of dengue virus (DENV-1, DENV-2, DENV-3, and DENV-4) and is for the people who have laboratory confirmed dengue infection and between the age of 9 to 16.
Present literature surveys were suggested that dengue is a highly dangerous viral disease which develops and spreaded in mammalian due to mosquito bite. Furthermore, dengue is a major issue all over the world. It more detailed molecular epidemiological data are needed particularly from those regions where the data of circulating DENV are not available. Such information is important not only to predict dengue outbreaks but also for the consideration of vaccine design and composition. Whatsmore compilation of data also reveals that molecular epidemiological studies, including Whole Genome Sequencing (WGS), is important in order to provide information on currently circulating viruses and to provide a better understanding of DENV transmission and epidemiology in a specific region. In regions where DENV co-circulating with other Arboviruses such as such CHIKV and ZIKV, prospective cohort studies that assessing those viruses will provide an informative picture of the transmission dynamics of these Arboviruses. More studies to investigate dengue pathogenesis are required, and the role of previous ZIKV infection on clinical outcomes should be further studied. It was seen that in 2022 recent outbreaks of dengue fever was in Nepal, Pakistan, and Bangladesh.
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Sahu, et al. 2023. A Systemic Review on Recent Outbreaks of Dengue and Newer US FDA Approved Drug. Der
Pharma Lett.15:01-05
Copyright: © 2023 Vinayak V. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.